Last Updated on September 23, 2025 by Muhamed Elmesery
Have you ever thought “What are the principal sources of energy on this planet, both natural or biological and artificial?” “What are the reactions that allow energy to be extracted from molecules?” “What is responsible for cellular respiration and photosynthesis?” “What is the cause of minerals formation and mobilization and changes in rocks colors?”
The answer is “Redox Reactions” which are the most important reactions in our life.
In this article, we will discuss the analytical laboratory method that depends on redox reaction, which is called “Redox Titration” and focus on applications of redox titration, principles, and types.
Redox reactions are all around us. In fact, much of our technology, from fire to laptop batteries, is largely based on redox reactions. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more complex process such as the oxidation of glucose (C6H12O6) in the human body through a series of electron transfer processes.
try now Redox Titration with praxilabs for free!
Table of Contents
Introduction
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of molecule, atom, or ion changes by gaining or losing an electron. Redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting.
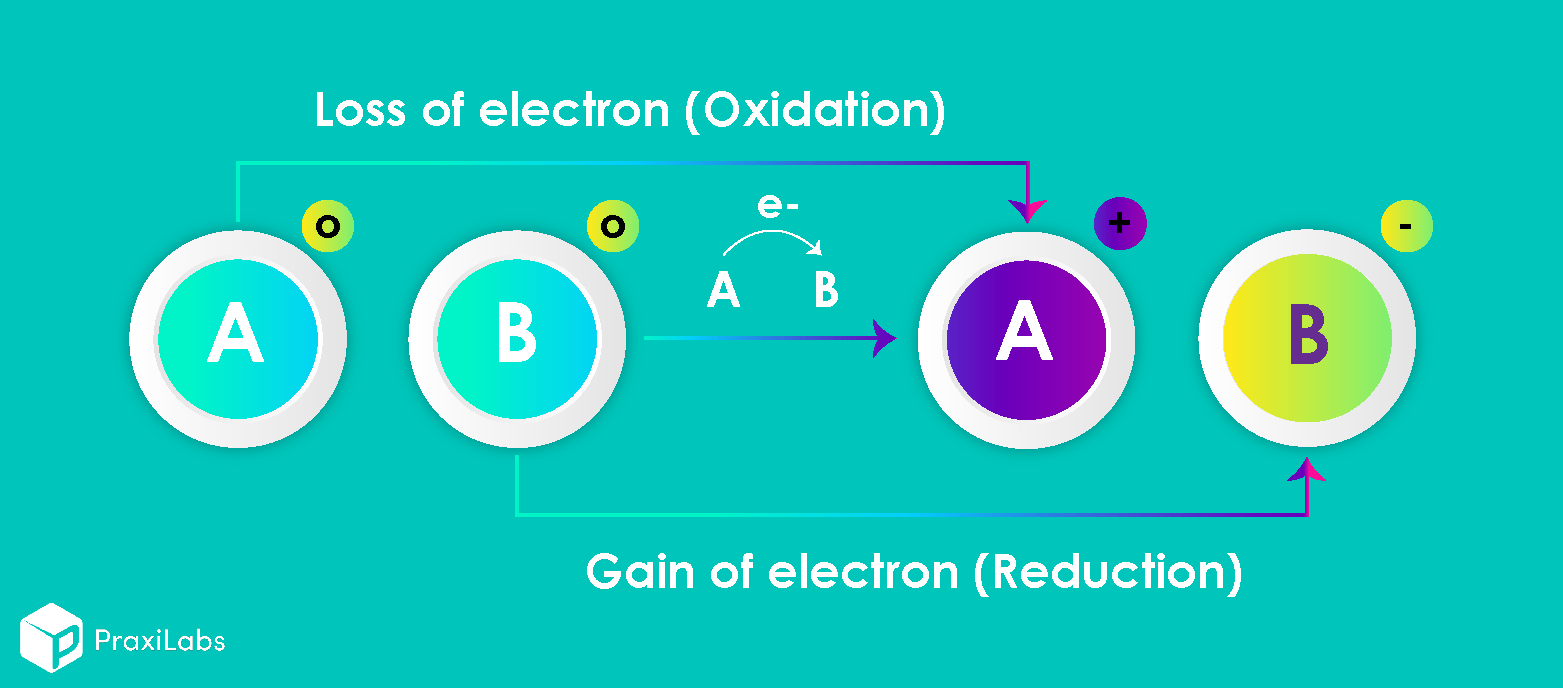
The two species that exchange electrons in a redox reaction are given special names. The ion or molecule that accepts electrons is called the oxidizing agent; by accepting electrons, it causes the oxidation of another species. Conversely, the species that donates electrons is called the reducing agent; when the reaction occurs, it reduces the other species. In other words, what is oxidized is the reducing agent and what is reduced is the oxidizing agent. (Note: the oxidizing and reducing agents can be the same element or compound, as in disproportionation reactions).
Concept of Oxidation and Reduction
Oxidation: It may be defined as a loss of electrons to an oxidizing agent (that undergoes reduction) to yield more positive or higher oxidation state.
Example:
Fe+2 (ferrous ion) into Fe+3 (ferric ion)
Reduction: It may be defined as gain of electrons from reducing agent (that undergoes oxidation) to give more snegative or lower oxidation state.
Example of Redox Reactions
In the reaction between hydrogen and fluorine, hydrogen is being oxidized and fluorine is being reduced:
H2 + F2 → 2 HF
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the weak, high-energy F-F bond. We can write this overall reaction as two half-reactions:
The oxidation reaction:
H2 → 2 H+ + 2 e−
And the reduction reaction:
F2 + 2 e− → 2 F−
Elements, even in molecular form, always have an oxidation state of zero. In the first half-reaction, hydrogen is oxidized from an oxidation state of zero to an oxidation state of +1. In the second half-reaction, fluorine is reduced from an oxidation state of zero to an oxidation state of −1.
When adding the reactions together, the electrons are canceled and the ions combine to form hydrogen fluoride:
2 H+ + 2 F− → 2 HF
The overall reaction is
H2 + F2 → 2 HF
Definition and Principle of Redox Titration
The redox titration is an oxidation-reduction reaction between an oxidizing agent and a reducing agent. In this type of titration, the chemical reaction takes place with a transfer of electrons in the reacting ions of aqueous solutions.
In the oxidation-reduction titration method, a reducing substance is titrated with standard solution of an oxidizing agent or an oxidizing substance is titrated with the standard solution of the reducing agent.
Redox titration may involve the use of a redox indicator and/or a potentiometer. A common example of a redox titration is treating a solution of iodine with a reducing agent to produce iodide using a starch indicator to help detect the endpoint.
The principle
involved in the oxidation-reduction titrations is that the oxidation process involves the loss of electrons whereas the reduction process involves the gain of electrons.
Oxidant + ne ↔ Reductant
Redox Titration Curve
To evaluate a redox titration we need to know the shape of its titration curve. In an acid–base titration or a complexation titration, the titration curve shows how the concentration of H3O+ (as pH) or Mn+ (as pM) changes as we add titrant. For a redox titration, it is convenient to monitor the titration reaction’s potential instead of the concentration of one species.
Try PraxiLabs Virtual Chemistry Lab for Free and Enjoy Science Education Anywhere and Anytime.
Try PraxiLabs Virtual Lab For FREE!
What are the types of Redox Titration ?
Types of Redox titrations are named according to the titrant that is used:
- Bromometry uses a bromine (Br2) titrant.
- Cerimetry employs cerium(IV) salts.
- Dichrometry uses potassium dichromate.
- Iodometry uses iodine (I2).
- Permanganometry uses potassium permanganate.
Now, we will discuss the types in detail
Permanganate Titrations

In this titration, the potassium permanganate is used as an oxidizing agent. It is maintained with the use of dilute sulfuric acid. Here is the equation:
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2 + 5O
Or MnO4– + 8H + 5e → Mn2+ + 4H2O
Further, the solution remains colorless before the endpoint. The potassium permanganate is used to estimate oxalic acid, ferrous salts, hydrogen peroxide, oxalates, and more. While the solution of potassium permanganate is always standardized before it is used.
you will find more details in our article Standardization of potassium permanganate in 7 steps.
Dichromate Titrations
These are titrations in which potassium dichromate is used as an oxidizing agent in acidic medium. The medium is maintained acidic by the use of dilute sulfuric acid. The potential equation is
K2Cr2O7 + 4H2SO4 → K2Cr2(SO4) + 4H2O + 3[O]
Or Cr2O27- + 14H + 6e → 2 Cr3+ + 7H2O
The solution of potassium dichromate can be directly used for titrations. It is mainly used for the estimation of ferrous salts and iodides.
Iodimetric and Iodometric Titrations

The reduction of free iodine to iodide ions and oxidation of iodide ions to free occurs in these titrations.
l2 + 2e → 2l–……………. (reduction)
2l– + 2e → 2e ……………. (oxidation)
The solution is used as an indicator. Free iodine is used in the iodometric titration, while in the iodometric titration an oxidation agent is used to react to liberate free iodine.
CERIMETRY
Cerimetry is a redox titration involving Cerric sulphate (Ce+4) as an oxidizing agent. Cerric sulfate is a powerful oxidizing agent and possesses a bright yellow colur; however during titration Cerric sulfate undergoes reduction to Cerrous sulfate (Ce+3) which is colorless in nature. And this marks the end point of titration.
Ce+4 + 1e———– Ce+3
BROMATOMETRY (POTASSIUM BROMATE TITRATION)

The specific titrations with potassium bromate are referred to as Bromatometry. It may be exploited as an effective and useful oxidizing agent in the qualitative determination (assay) of pharmaceutical substances like mephenesin, phenol sodium and salicylate. It can also be used for the analysis of organic arsenicals like carbasone (C7H9AsN2O4).
Principle: The fundamental underlying principle of ‘Bromatometry’ exclusively and predominantly depends upon the formation of iodine monobromide [IBr] in relatively higher actual strength of HCl solution.
We can also classify the types of redox titration according to the processes involved in a redox reaction and describe what happens to their various components:
Try redox titration with 3D Virtual Labs Now
Combination
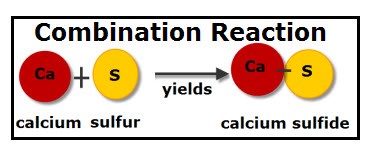
Combination reactions “combine” elements to form a chemical compound. As usual, oxidation and reduction occur together.
For example:
2 H2 + O2 → 2 H2O
The sum of oxidation states in the reactants is equal to that in the products: 0 + 0 → (2)(+1) + (-2)
In this equation, both H2 and O2 are the molecular forms of their respective elements and therefore their oxidation states are 0. The product is H2O: the oxidation state is -2 for oxygen and +1 for hydrogen.
Decomposition
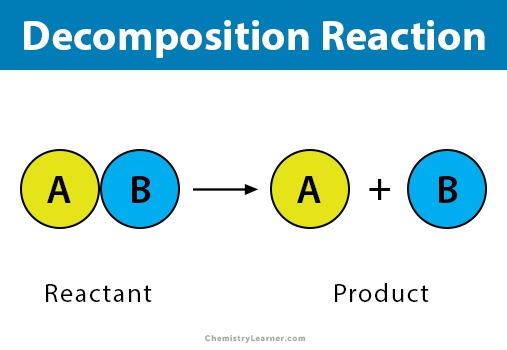
Decomposition reactions are the reverse of combination reactions, meaning they are the breakdown of a chemical compound into its component elements.
For example:
2 H2O → 2 H2 + O2
(2)(+1) + (-2) = 0 → 0 + 0
In this equation, the water is “decomposed” into hydrogen and oxygen, both of which are neutral. Similar to the previous example, H2O has a total oxidation state of 0, with each H taking on a +1 state and the O a -2; thus, decomposition oxidizes oxygen from -2 to 0 and reduces hydrogen from +1 to 0.
Displacement
Displacement reactions, also known as replacement reactions, involve compounds and the “replacing” of elements. They occur as single and double replacement reactions.
- A single replacement reaction “replaces” an element in the reactants with another element in the products.
For example:
Cl2 + 2 NaBr → 2 NaCl + Br2
In this equation, Cl is reduced and replaces Br, while Br is oxidized.
- A double replacement reaction is similar to a single replacement reaction but involves “replacing” two elements in the reactants with two in the products.
For example:
Fe2O3 + 6 HCl → 2 FeCl3 + 3 H2O
In this equation, Fe and H as well as O and Cl trade places.
Combustion
Combustion reactions always involve oxygen and an organic fuel. In the following image, we see methane combusting to release energy.
Combustion Reaction of Methane
This is an example of a combustion reaction, a redox process. Methane reacts with oxygen to form carbon dioxide and two water molecules.
Disproportionation
In some redox reactions, substances can be both oxidized and reduced. These are known as disproportionation reactions. One real-life example of such a process is the reaction of hydrogen peroxide, H2O2, when it is poured over a wound. At first, this might look like a simple decomposition reaction, because hydrogen peroxide breaks down to produce oxygen and water:
2 H2O2 (aq.) → 2 H2O(l) + O2(g)
The key to this reaction lies in the oxidation states of oxygen, however. Notice that oxygen is present in the reactant and both products. In H2O2, oxygen has an oxidation state of -1. In H2O, its oxidation state is -2, and it has been reduced. In O2,however, its oxidation state is 0, and it has been oxidized. Oxygen has been both oxidized and reduced in the reaction, making this a disproportionation reaction.
Applications of Redox Titration
Redox reactions are all around us. In fact, much of our technology, from fire to laptop batteries, is largely based on redox reactions. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more complex process such as the oxidation of glucose (C6H12O6) in the human body through a series of electron transfer processes.
-
Applications of Redox Titration in Chemistry

It is usually used to determine medium and high concentrations of elements. Furthermore, titration gives reliable results even in field conditions. Redox titrimetry is used to analyse a wide range of inorganic analytes. A redox titration (also called an oxidation-reduction titration) can accurately determine the concentration of an unknown analyte by measuring it against a standardized titrant.
It is used for the analysis of organic analytes. One important example is the determination of the chemical oxygen demand (COD) of natural waters and wastewaters.
Get Started Praxilabs For FREE!
-
Redox Titration Real Life Applications
- Production of some important chemicals is also based on electrolysis which in turn is based on redox reactions. Many chemicals like caustic soda, chlorine, etc. are produced using redox reactions.
- Oxidation-Reduction reactions also find their application in sanitizing water and bleaching materials.
- The surfaces of many metals can be protected from corrosion by connecting them to sacrificial anodes which undergo corrosion instead. A common example of this technique is the galvanization of steel.
- The industrial production of cleaning products involves the oxidation process.
- Nitric acid, a component of many fertilizers, is produced from the oxidation reaction of ammonia.
- Electroplating is a process that uses redox reactions to apply a thin coating of a material on an object. Electroplating is used in the production of gold-plated jewelry.
- Many metals are separated from their ores with the help of redox reactions. One such example is the smelting of metal sulfides in the presence of reducing agents.
- The main source of oxidation is oxygen and therefore redox reaction or oxidation-reduction reactions are responsible for food spoilage.
- Combustion is a type of oxidation-reduction reaction and hence it is a redox reaction. An explosion is a fast form of combustion and hence explosion can be treated as a redox reaction. Even the space shuttle uses redox reactions. The combination of ammonium perchlorate and powdered aluminium inside the rocket boosters gives rise to an oxidation-reduction reaction.
-
Applications of Redox Titration in Pharmacy
Redox titration is used in pharmaceutical analysis like in the determination of valganciclovir hydrochloride (VLGH) in pure drugs and tablets.
Two simple, selective and sensitive spectrophotometric methods were developed and validated.
The first method was based on the reduction of iron(III) to iron(II) by VLGH and subsequent formation of iron(III)-ferricyanide complex (Prussian blue) in acid medium which was measured at 730 nm (method A).
In the second method (method B), permanganate was reduced by VLGH to bluish green manganate in alkaline medium and the absorbance was measured at 610 nm. The absorbance measured in each case was related to VLGH concentration.
- Applications of Redox Titration in Industry
- Content analysis, wherein redox (oxidation-reduction) reactions are used to establish the purity of raw materials, including binding substances in oral medications, rather than the end product itself.
- One of the most important industrial applications of redox titrations is evaluating the chlorination of public water supplies. Representative Method 9.3, for example, describes an approach for determining the total chlorine residual by using the oxidizing power of chlorine to oxidize I– to I3–. The amount of I3– is determined by back titration with S2O32–.
- We can also find application of redox titration in polymer.
-
Applications of Redox Titration in Public Health and Environmental Analyses
The determination of dissolved oxygen. In natural waters, such as lakes and rivers, the level of dissolved O2 is important for two reasons: it is the most readily available oxidant for the biological oxidation of inorganic and organic pollutants; and it is necessary for the support of aquatic life. In a wastewater treatment plant dissolved O2 is essential for the aerobic oxidation of waste materials. If the concentration of dissolved O2 falls below a critical value, aerobic bacteria are replaced by anaerobic bacteria, and the oxidation of organic waste produces undesirable gases, such as CH4 and H2S.
-
Applications of Redox Titration in Food

Titration is an analytical technique that is widely used in the food industry. It allows food manufacturers to determine the quantity of a reactant in a sample. It can be used to discover the amount of salt or sugar in a product or the concentration of vitamin C or E, which has an effect on product color.
For example:
- Determination of salt in cheese and butter.
Reaction of salt in food with standard solution of silver nitrate
AgNO3 +NaCl ——– AgCl + NaNO3
Unreacted AgNO3 is titrated with potassium thiocyanate using Fe3+ as an indicator.
AgNO3 + KCNS —— AgCNS + KNO3
The endpoint reacts with Fe3+ to produce reddish brown precipitate when all salt is reacted.
-
Applications of Redox Titration in Dentistry
In the case of teeth whitening, hydrogen or carbamide peroxide are the active ingredients in the whitening agent. They are highly similar in chemical composition, as carbamide peroxide breaks down into hydrogen peroxide when applied to the teeth. During a teeth whitening procedure, it is the hydrogen peroxide that becomes oxidized and breaks the double chemical bonds of the chromogens, scattering their molecules. Because stains result from accumulations of chromogens, once scattered they appear lighter in color.
-
Applications of Redox Reaction in Electrochemistry
The battery used for generating DC current uses redox reaction to produce electrical energy. Batteries or electrochemical cells used in our day-to-day life are also based on redox reactions. For example, storage cells are used in vehicles to supply all the electrical needs of the vehicles.
-
Applications of Redox Titration in Metallurgy
It is used in metallurgical processes for extracting metals from ores and combustion of fuel. One of the titration methods used in hydrometallurgy is an oxidation-reduction (redox) titration.
Get Start Now For Free
PraxiLabs provides virtual experiments simulations in analytical chemistry.. Create a free account now and try our simulations that you can access anytime and anywhere to perform a variety of chemistry experiments.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science






