Last Updated on May 15, 2025 by Muhamed Elmesery
Chemistry is the branch of science where interactions and reactions between elements, atoms, electrons, molecules, and many other particles, is mainly considered and studied.
One of the most famous reactions widely abundant and occurring in chemistry, and in our day-to-day life, is the topic of this article: the process of oxidation and reduction and their reactions.
Here, we will discuss the nature of these reactions, their definitions, the difference between oxidation potential and reduction potential, and the difference between oxidizing agents and reducing agents, in addition to giving oxidation vs. reduction examples to clearly comprehend the process… Let’s start!
try Oxidation and Reduction Reactions at Praxilabs for FREE
Table of Contents
Oxidation and Its Historical Definition
In case you are a fan of oxygen-based cleansers, or you are thankful for the sterilizing power of hydrogen peroxide products, then you must thank “oxidation.” On the other hand, you can blame oxidation, if you ever have dealt with a rusty car or a toss out browned fruit.
Oxidation is a process that can start spontaneously or artificially; it is helpful at times and very destructive at others.

The very classical example of oxidation is when iron interacts and combines with oxygen to form the iron oxide, or the famous reddish or orangish component: rust.
On PraxiLabs you can find different virtual labs simulations accessible anytime and anywhere. Subscribe and Get Started NOW!
In this example, iron is said to be oxidized into rust, where the chemical reaction is given by:
2 Fe + O2 → Fe2O3
The older definition of the oxidation process was defined when oxygen or any other electronegative element was added to a compound. This very basic understanding was originating because oxygen (O2) was the first oxidizing agent to be known.
Later on, oxidation’s definition was widened to include other types of chemical reactions that do not necessarily contain oxygen. Nevertheless, oxygen’s addition to a compound meets the criteria of electron loss perfectly, in parallel with an increase in the oxidation state.
Oxidation Definition
Nowadays, the term oxidation is not looked at to be only oxygen-related, but it is looked at as a whole process that does not have to include oxygen. Rather, oxidation is defined as:
“the loss of electrons during a reaction by a molecule, atom, or ion.”
Not all elements have the same tendency to lose or gain electrons. Some elements, i.e., metals, including magnesium, iron, and sodium, are easily oxidized, whereas the nonmetals like, chlorine, nitrogen, and oxygen, are not easily oxidized, and they are more reluctant to lose their electrons.
Oxidation reactions may involve many forms, as follows:
- Addition of oxygen
C + O2 → CO2 (oxidation of carbon) - Addition of an electronegative element
Fe + S → FeS (oxidation of iron)
- Removal of an electropositive element
2 KI + H2O2 → I2 + 2 KOH (oxidation of iodide) - Removal of hydrogen
H2S + Br2 → 2 HBr + S (oxidation of sulfide)
Another important definition is the oxidizing agent, which is:
“a substance that accepts, gains, or receives an electron from a reducing agent.”
Or, more briefly,
“Any substance that oxidizes another substance.”
In the four previously mentioned examples, the oxidizing agents are: O2, S, Br2, H2O2.
What is Oxidation Mechanism ?
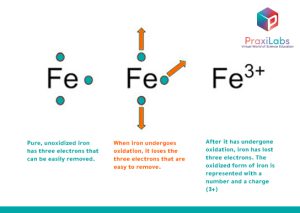
Oxidation occurs in stages, and it results in a change in the properties of the atom or the compound that is being oxidized. For example, when iron experiences oxidation, it is transformed into a brittle, reddish powder, and it loses its stiffness and structurally-sound metal nature. This happens as a consequence of the process of losing electrons.
To visually imagine what happens clearly, this following diagram illustrates what happens to an iron atom as it is oxidized. It starts to carry a charge once it is oxidized. This charge is:
- Not only one, but 3 charges, and
- Positive, as it lost 3 electrons.
This is chemically denoted by (3+) sign written as a superscript to the right of the iron (Fe) symbol. Iron is an effortlessly oxidized element; that is, iron’s exposure to oxygen and moisture is important and is always preferred. As long as oxygen is abundant, iron will keep losing its electrons.
Try oxidation reduction at our virtual chemistry labs
Examples of Oxidation Reactions
Many reactions are considered as great examples of oxidation reactions, such as:
- The reaction between hydrogen and fluorine to give out hydrofluoric acid, that is given by:
H2 + F2 → 2 HF This reaction can be better understood if it is written in terms of two half reactions, like:
H2 → 2 H++ 2 e–
F2 + 2e– → 2 F–
It is easily noticed that this reaction does not have any oxygen atoms in any of its parts. - The interaction between copper and silver is a great example of electrochemical reactions, where:
Cu (s) + 2 Ag+ (aq) → Cu2+ (aq) + 2 Ag (s)Here, a wire of copper is placed into a silver ions solution, where electrons transfer from the copper metal to the silver ions. As a consequence, copper is oxidized by releasing its ions into the solution, and silver whiskers grow onto the copper wire. - The reaction between magnesium and oxygen to give out magnesium oxide is one example of oxidation where oxygen is conspicuous in the equation. That is to say: 2 Mg (s) + O2 (g) → 2 MgO (s)
Reduction Definition
The counter process to that of oxidation is known as reduction, and rationally speaking, it is the process of gaining electrons. Reduction is defined as:
“The gain of electrons during a reaction by a molecule, atom, or ion.”
The historical perspective of reduction was viewed as if it is a process where hydrogen, or any electropositive element, is added.
Reduction Mechanism
Reduction mechanism is not any different than that of the oxidation. Here, and instead of losing electrons as it is the case with oxidation, elements gain electrons, and the compound is said to be reduced.
Also, changes happen to the properties of the atom or the compound that is being reduced.
Examples of Reduction Reactions
This may involve many forms, as follows:
- Addition of hydrogen
N2 + 3 H2 → 2 NH3 (reduction of nitrogen) - Addition of electropositive element
SnCl2 + 2 HgCl2 → SnCl4 + HgCl2 (reduction of mercuric oxygen) - Removal of oxygen
ZnO + C → Zn + CO (reduction of zinc oxide) - Removal of electronegative element
2 FeCl3 + H2 → 2 FeCl2 + 2 HCl (reduction of ferric chloride)
Again, the reduction agent is important to be defined as follows:
“a substance that gives, loses, or donates an electron from an oxidizing agent.”
Or, more briefly,
“Any substance that oxidizes another substance.”
The reduction agents in the previous examples are: H2, HgCl2, and C.
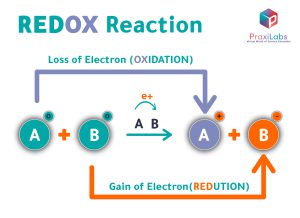
Oxidation and reduction are two simultaneous processes that occur together, and they result in a very famous reaction, known as: redox reaction. In which, the atoms or the compounds gain and lose electrons simultaneously at the same time.
Join PraxiLabs for FREE, and enjoy your oxidation-reduction simulation!
Identifying Oxidizing and Reducing Agents
An Oxidizing agent is a substance that accepts, gains, or receives an electron from a reducing agent.
A reducing agent is a substance that gives, loses, or donates an electron from an oxidizing agent.
For identifying oxidizing and reducing agents:
Break the reaction down into a net ionic equation and then into half-reactions.
The substance that loses electrons is being oxidized and is the reducing agent.
The substance that gains electrons is being reduced and is the oxidizing agent.
For more clarification, let’s take an example:
When chlorine gas is bubbled into a solution of sodium bromide, a reaction occurs which produces aqueous sodium chloride and bromine. Determine what is being oxidized and what is being reduced. Identify the oxidizing and reducing agents.
Cl2(g)+2NaBr(aq)→2NaCl(aq)+Br2(l)
Break the reaction down into a net ionic equation and then into half-reactions.
Cl2(g)+2Na+(aq)+2Br−(aq) -→ 2Na+(aq)+2Cl−(aq)+Br2(l)
Cl2(g)+2Br−(aq) →2Cl−(aq)+Br2(l) (net ionic equation)
Reduction: Cl2(g)+2e−→2Cl−(aq)
Oxidation: 2Br−(aq)→Br2(l)+2e−
The Cl2 is being reduced and is the oxidizing agent.
The Br− is being oxidized and is the reducing agent.
Another example:
Write the following reaction in the form of half-equations. Identify each half-equation as an oxidation or a reduction. Also identify the oxidizing agent and the reducing agent in the overall reaction
Zn+2Fe3+⟶Zn2++2Fe2+
Answer
The half-equations are
Zn⟶Zn2++2e− oxidation—loss of electrons
2e−+2Fe3+⟶2Fe2+ reduction—gain of electrons
Zinc has been oxidized; the oxidizing agent must be the other reactant, namely, iron(III).
Iron(III) ions have been reduced; the zinc must be the reducing agent.
Source : Libretexts (2022) 8.2: Oxidizing and reducing agents, Chemistry LibreTexts. Available at: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Changing_Times_(Hill_and_McCreary)/08%3A_Oxidation_and_Reduction/8.02%3A_Oxidizing_and_Reducing_Agents (Accessed: 28 July 2024).
Note: For identifying reduced and oxidized Elements, you should know that the elements that lose electrons are said to be oxidized, while the elements that gain electrons are said to be reduced.
Oxidation and Reduction Demystified: Your FAQs Answered
What are the 4 types of oxidation- reduction reactions?
There are 4 types of oxidation-reduction reactions (Redox Reactions):
- Combination Reactions.
- Decomposition Reactions.
- Displacement Reactions.
- Disproportionation Reactions.
Combination
Combination reactions “combine” elements to form a chemical compound. As usual, oxidation and reduction occur together.
For example:
2 H2 + O2 → 2 H2O
The sum of oxidation states in the reactants is equal to that in the products: 0 + 0 → (2)(+1) + (-2)
Decomposition
Decomposition reactions are the reverse of combination reactions, meaning they involvethe breakdown of a chemical compound into its component elements.
For example:
2 H2O → 2 H2 + O2
(2)(+1) + (-2) = 0 → 0 + 0
Displacement
Displacement reactions, also known as replacement reactions, involve compounds and the “replacement”of elements. They occur as single and double replacement reactions.
- A single replacement reaction “replaces” an element in the reactants with another element in the products.
For example:
Cl2 + 2 NaBr → 2 NaCl + Br2 (Cl is reduced and replaces Br, while Br is oxidized)
- A double replacement reaction is similar to a single replacement reaction but involves “replacing” two elements in the reactants with two in the products.
For example:
Fe2O3 + 6 HCl → 2 FeCl3 + 3 H2O (Fe and H as well as O and Cl trade places)
Disproportionation
Disproportionation reactions refer to the reactions of some substances which can be both oxidized and reduced. For example: the reaction of hydrogen peroxide, H2O2, when it is poured over a wound. At first, this might look like a simple decomposition reaction, because hydrogen peroxide breaks down to produce oxygen and water:
2 H2O2 (aq.) → 2 H2O(l) + O2(g)
The key to this reaction lies in the oxidation states of oxygen, however. Notice that oxygen is present in the reactant and both products. In H2O2, oxygen has an oxidation state of -1. In H2O, its oxidation state is -2, and it has been reduced. In O2,however, its oxidation state is 0, and it has been oxidized. Oxygen has been both oxidized and reduced in the reaction, making this a disproportionation reaction.
What is oxidation vs reduction reactions in organic chemistry?
| Oxidation Reaction | Reduction Reaction |
| Oxidation is the loss of electrons during a reaction by a molecule, atom, or ion.
Oxidation is the process by which a carbon atom gains bonds to more electronegative elements, with oxygen being the most common. |
Reduction is the gain of electrons during a reaction by a molecule, atom, or ion.
Reduction is a process by which a carbon atom gains bonds to less electronegative elements, with hydrogen being the most common. |
There are many forms of oxidation reactions:
|
There are many forms of reduction reactions:
|
| Oxidizing agent is a substance that accepts, gains, or receives an electron from a reducing agent. | Reduction agent is a substance that gives, loses, or donates an electron from an oxidizing agent. |
What are examples of oxidation?
- Rusting is a form of corrosion of metal. Corrosion occurs due to the degradation of a metal by the action of water (moisture) and air as a result of the metal oxidation. Iron catches rust because it gets oxidized in the presence of air and water to form hydrated iron oxide (Fe2O3.xH2O).
- The iron oxide forms a reddish-brown covering on the metal surface. 4Fe + 3O2 + 2xH2O —> Fe2O3.xH2O (Rust).
- The rancidity happens when fried foods acquire a bad odor and flavor after being exposed to air for a long time.
- The respiration is the process that happens inside our bodies, which triggers an oxidation reaction. The food is oxidized during the metabolism cycles to produce the energy we need for our life.
- Browning: Oxygen in the air can cause the sliced fruits )such as apple( to brown, a process called enzymic browning which is considered as an oxidation reaction. In the apple cells, phenols and the enzyme phenolase are found, and when exposed to oxygen in the air, for example through slicing, the oxygen causes a reaction (oxidation).
- The interaction between copper and silver is a great example of electrochemical reactions, where:
Cu (s) + 2 Ag+ (aq) → Cu2+ (aq) + 2 Ag (s) . Here, a wire of copper is placed into a silver ions solution, where electrons transfer from the copper metal to the silver ions. As a consequence, copper is oxidized by releasing its ions into the solution, and silver whiskers grow onto the copper wire.
- Combustion reactions. For example, Bunsen burners which are used in the school lab use methane (a hydrocarbon) as fuel, which burns with oxygen in the air (oxidation reaction).
How can a reaction be both oxidation and reduction?
The reaction can be both oxidation and reduction in case of redox reactions, which is a type of chemical reaction that involves a transfer of electrons between two species.
In redox reactions, oxidation and reduction are two simultaneous processes that occur together, and the atoms or the compounds gain and lose electrons simultaneously at the same time.
Example of Redox Reactions
In the reaction between hydrogen and fluorine, hydrogen is being oxidized and fluorine is being reduced:
H2 + F2 → 2 HF
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the weak, high-energy F-F bond. We can write this overall reaction as two half-reactions:
The oxidation reaction:
H2 → 2 H+ + 2 e−
And the reduction reaction:
F2 + 2 e− → 2 F−
Elements, even in molecular form, always have an oxidation state of zero. In the first half-reaction, hydrogen is oxidized from an oxidation state of zero to an oxidation state of +1. In the second half-reaction, fluorine is reduced from an oxidation state of zero to an oxidation state of −1.
When adding the reactions together, the electrons are canceled and the ions combine to form hydrogen fluoride:
2 H+ + 2 F− → 2 HF
The overall reaction is
H2 + F2 → 2 HF
PraxiLabs provides virtual experiments simulations in analytical chemistry.. Create a free account now and try our simulations that you can access anytime and anywhere to perform a variety of chemistry experiments.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





