Last Updated on October 11, 2025 by Muhamed Elmesery
The sulfite ion is the conjugate base of bisulfite. It is a sulfur oxoanion (Sulfite ion is one of the oxyanions of sulfur), a sulfur oxide and a divalent inorganic anion. It is a conjugate base of a hydrogen sulfite.
Although sulfonic acid is not commonly available, its salts are highly abundant naturally in many foods and are commonly utilized as food additives. Sulfite ion, also written as sulphite, is an ion that is present in many binary salts largely used in chemical industries.
Get started Praxilabs for FREE
Table of Contents
Know All about Sulfite Ion Structure, Formula, and Charge
Sulfite ion is formed by one centered sulfur cation S4+ and three oxygen anions O2-.
The geometry is a trigonal pyramid with the three oxygen atoms forming the base and over the sulfur atom, there is a lone pair of electrons.
The molar mass is 80.06 g/mol.
What Is the Formula for Sulfite Ions?
The chemical formula for sulfite ion is SO₃²⁻
Sulfite Ion Structure!
Its chemical structure can be written as
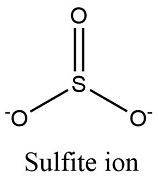
Master Everything about Sulfite Ion Charge
A sulfite is an ion that is made of one sulfur atom and three oxygen atoms; it has a charge of two minus (-2).
Lewis Structure for Sulfite Ion: How Do You Draw It?
The following steps are required to draw the sulfite ion Lewis structure:
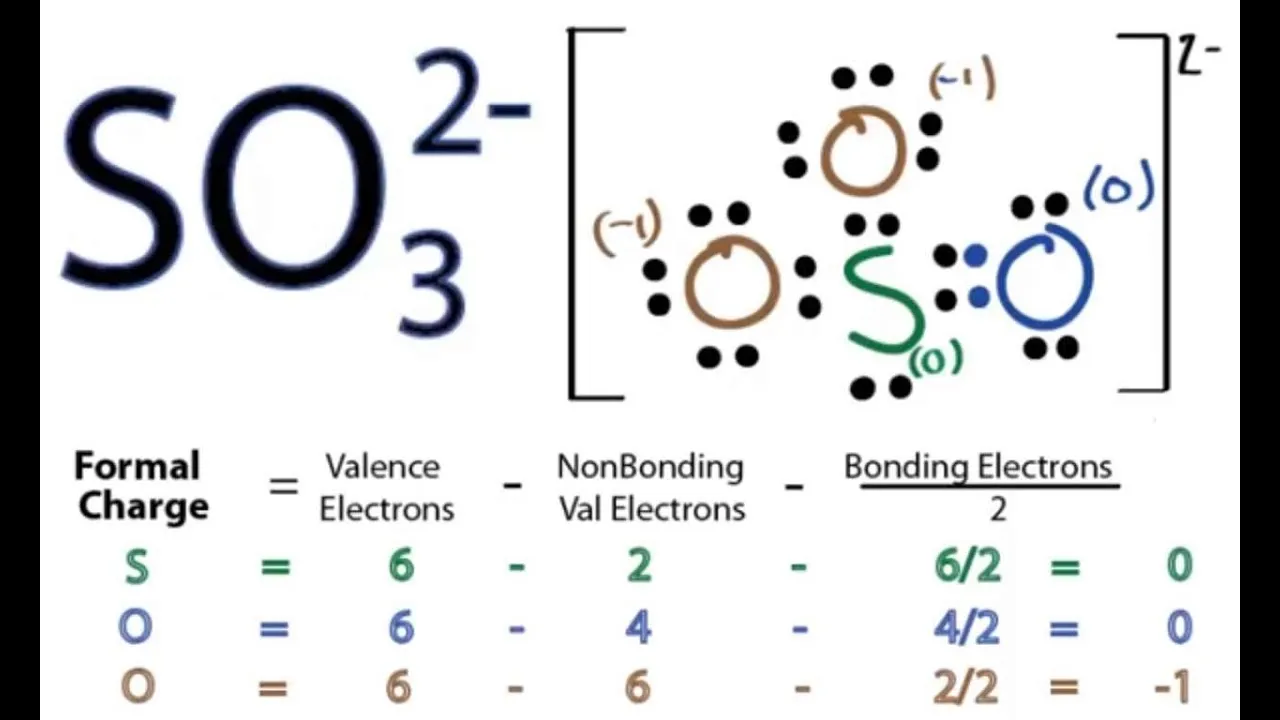
- Find total number of electrons of the valence shells of sulfur and oxygen atoms.
- Find the total electrons pairs.
- Select the Center atom.
- Put lone pairs on atoms.
- Check the stability and minimize charges on atoms by converting lone pairs to bonds.
Sulfite Ion Lewis Structure Figure
The following figure shows how to draw sulfite ion Lewis dot structure:
Video shows how to draw Lewis Structures in five steps
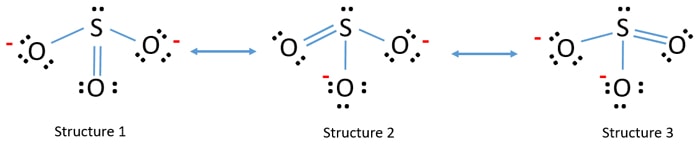
Sulfite Ion Resonance Structures
Sulfite anion possesses three equivalent resonating structures. Each of them has its sulfur atom bonded with one of its oxygen atoms via a double bond and possesses a zero formal charge (neutral). The sulfur atom is bonded to the other two oxygen atoms through single bonds, where each oxygen atom carries a formal charge of 1− indicating the 2− charge on the entire sulfite anion. A non-bonded lone pair of electrons is located on the sulfur atom of the sulfite. Therefore, according to the VSEPR theory, the sulfite anion possesses a trigonal pyramidal configuration like ammonia (NH3).
Change the location of double bond and lone pairs of molecule to draw resonance structures of SO32- ion. The hybrid resonating structure of the sulfite anion possesses three equivalent S−O bonds as shown below:
Hydrogen Sulfite Ion:
Hydrogen sulfite is a sulfur oxoanion. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, and a mouse metabolite. It is a conjugate base of a sulfurous acid. It is a conjugate acid of sulfite.
Sulfite Ion Chemical Properties and Reactions
The sulfite is a stable ion as three oxygen atoms are bound through a double and two single bonds, but it remains as a resonant structure in which the three atoms are bound through a bond that has characteristics of single and double bonds. This structure can stabilize the two negative charges, making the ion stable.
Although sulfite ion is counted as a weak base, it undergoes hydrolysis to produce basic solutions.
SO32− (aq) + H2O (l) ↔ HSO3− (aq) + OH− (aq)
In the presence of an acidic solution, the equilibrium is shifted towards formation of sulfurous acid, leading to sulfur dioxide (SO2) gas evolution. Sulfur dioxide is a colorless gas with a characteristic pungent odor.
HSO3− (aq) + 2 H2O (l) ↔ H2SO3 (aq) + OH− (aq)
H2SO3 (aq) ↔ H2O (l) + SO2 (g)
Sulfite ions get oxidized easily upon exposure to oxygen in air.
2 SO32− (aq) + O2 (g) ↔ 2 SO42− (aq)
Therefore, sulfite and sulfur dioxide could decolorize the permanganate solution. Therefore, the reaction can be used to test for sulfur dioxide.
2MnO4− (aq) + 5SO2 (g) + 2H2O (l) ↔ 5SO42− (aq) + 2Mn2+ (aq) + 4H+ (aq)
The sulfite ion is classified as a member of acidic radicals of the first group in which hydrochloric acid is used as the group reagent. Hydrochloric acid displaces sulfite ions in their salts leading to liberation of sulfur dioxide SO2 gas that could be detected using potassium dichromate paper.
In addition, soluble sulfite salts such as potassium sulfite could be detected through some confirmatory tests using silver nitrate solutions or lead acetate solutions since they react together forming silver sulfite or lead sulfite, respectively as a white precipitate. This returns back to the low solubility product of silver and lead sulfite salts so they precipitates very easily at very low concentrations.
Iodine and permanganate tests are carried out as specific tests for detection of sulfite ions, where the sulfite ions decolorize both solutions owing to their reducing activity.
Test for Sulfite Ion From PraxiLabs
Fascinating Things to Know about It!
What’s the General Aim of Sulfite Ion Test?
Detection of the presence of sulfite ion as an acid radical in inorganic salts such as potassium sulfite.
Top 6 Learning Objectives of Sulfite Ion Test
- Define and differentiate between sulfite ions and other acid radicals through their chemical formulas.
- Classify inorganic salts according to their acid radicals.
- Compare between sulfite and other first group members in terms of chemical structures, properties and reactions.
- Identify sulfite radicals containing salts experimentally.
- Select the appropriate reagents to detect the presence of sulfite radical.
- Balance the chemical equations of chemical reactions.
Method: Sulfite Ion Test
Detection of the presence of sulfite as acid radical using specific chemical reagents.
Principle of Work in Sulfite Ion Test
In this experiment, sulfite ion in potassium sulfite is detected through some identification and confirmatory tests. In addition, these tests can be used to differentiate between the first anionic class and other acid radical classes. Moreover, confirmatory and specific tests are used to differentiate between presences of different members of the first class of anions.
Learn 6 General Steps in Sulfite Ion Test
First: Solubility Test: In this test, a sample of the sulfite salt is tested for its solubility in distilled water on cold. Most sulfite salts are water insoluble except sodium, potassium and ammonium sulfites which are soluble in water without need of heating.
Second: Hydrochloric Acid Test: It depends on the fact that hydrochloric acid can displace sulfite ions in its potassium salt forming potassium sulfite salt and sulfur dioxide gas. The evolved gas can react with potassium dichromate paper causing it to change its color into green. The two steps of the reactions are:
Step 1: Reaction of Potassium Sulfite with Hydrochloric Acid:
K2SO3 + 2HCl → 2KCl + H2O + SO2 ↑
Step 2: Reaction of SO2 gas with Potassium Dichromate:
3SO2 + K2Cr2O7 + H2SO4 → K2SO4 + Cr2(SO4)3 + H2O
Third: Silver Nitrate Test:
Silver nitrate solution is added to a solution of potassium sulfite leading to the precipitation of silver sulfite salt as a white precipitate due to its low solubility product. The reaction of the test is:
K2SO3 + 2 AgNO3 → 2 KNO3 + Ag2SO3↓
Fourth: Lead Acetate Test:
Lead acetate solution is added to potassium sulfite solution resulting in precipitation of lead sulfite as a white precipitate due to low solubility product as shown in the following chemical reaction:
K2SO3 + Pb(CH3COO)2 → 2 CH3COOK + PbSO3 ↓
Fifth: Iodine Test:
Iodine test is considered as a specific test for detection of the presence of sulfite ion as the acid radical of a salt. The test depends on the reducing ability of sulfite salt to reduce iodine solution into hydrogen iodide leading to discoloration of its brown color.
K2SO3 + I2 + H2O → K2SO4 + 2HI
Sixth: Potassium Permanganate Test:
Permanganate test is considered as a specific test for detection of the presence of sulfite ion as the acid radical of a salt. The test depends on the reducing ability of sulfite salt to reduce permanganate MnO4– into manganous Mn2+ leading to discoloration of its purple color.
5K2SO3 + 2KMnO4 + 3H2SO4 → 6K2SO4 + 2MnSO4 + 3H2O
PraxiLabs provides a Test for Sulfite Radical Virtual Lab Simulation that you can access anytime and anywhere to perform it. Subscribe and get start now!
Health Effects of Sulfite Ion
Sulfite ions are not flammable and in general, moderate consumption of these salts is not dangerous for health, although it can be reported as one of the most allergic preservatives.
Topical, oral, or parenteral exposure to sulfites has been reported to induce a range of adverse clinical effects in sensitive individuals, ranging from dermatitis, urticaria, flushing, hypotension, abdominal pain, and diarrhea to life-threatening anaphylactic and asthmatic reactions.
Exposure to the sulfites arises mainly from the consumption of foods and drinks that contain these additives; however, exposure may also occur through the use of pharmaceutical products, as well as in occupational settings.
9 Mind Blowing Applications of Sulfite Ion (Sulfite Ion Uses)
Sulfite is used in:
- The photography industry to protect developing solutions from oxidation
- The pulp and paper industry
- Water treatment as an oxygen scavenger agent
- The leather industry as a desulfurizing and dechlorinating agent
- Textile industry as a bleaching agent
- Sodium sulfite is a component in many pharmaceuticals, which is effective to maintain the potency and stability of drugs. It is added to a number of drug preparations as an antioxidant and antimicrobial agen
- Sulfite is used as a food preservative, such as calcium sulfite, potassium sulfite, and sodium sulfite. They are used as a dried agent too, particularly for fruits
- Some sulfites are also used to produce the caramel color food coloring
- Sulfites occur naturally in all wines to some extent] Sulfites are commonly introduced to arrest fermentation at a desired time, and may also be added to wine as preservatives to prevent spoilage and oxidation at several stages of the winemaking. Sulfur dioxide (SO2) protects wine not only from oxidation, but also from bacteria
Sulfite Analysis Ion Chromatography
Sulfite analysis is a necessity for foods and beverages to be compliant with various food labeling regulations. Most foods and beverages contain residual sulfite concentrations within a range of approximately 10–2000 mg/kg.
Many individuals are sensitive to sulfite additives and some have experienced mild to severe allergic reactions, however, the exact mechanism behind this remains unknown, despite several hypotheses tested. Therefore, both U.S. Food and Drug Administration (FDA) and European Union (EU) laws require that the presence of sulfites be declared on food labels when the sulfite concentration is higher than 10 mg/L. For certain people, sulfite exposure in food has been reported to induce asthmatic episodes, though further tests have been performed to determine how this affects the asthmatic population specifically, as this sensitivity may have been overestimated in previous studies.
Ion chromatography (IC) is a sensitive and selective analytical technique for the determination of free and total sulfites in food was developed with electrochemical detection.
The IC technique also provides a wealth of additional information, such as:
- Sulfite and sulfate (oxidized sulfite) content of the spiking or treatment solution.
- Residual sulfite applied to the food after oxidation losses in the treatment process.
- Free sulfite in foods.
- Total sulfite in foods.
As a further check on the Monier-Williams method, the sulfate content of the trapping solution can be determined by IC. Because the IC technique traps the liberated SO2 in a non-oxidizing rather than an oxidizing medium, it is considered free from interfering sulfides and organic sulfur-containing groups which can give false positives in the Monier-Williams method. IC thus offers a high speed, more sensitive, and cost-effective alternative to conventional techniques for the determination of sulfite in foods.
- Ion chromatography (IC) is shown to be a promising technique for the determination of sulfites in foods. Results of a 10 min flash distillation and 10 min IC determination compare favorably with the results from the conventional Monier-Williams method for total sulfite in a variety of food matrices.
- Free and total sulfites in dehydrated foods were analyzed by extraction of the sulfites at pH 2.0 and 8.9, respectively, and separation on an anion exclusion column with 5 mM H2SO4, pH 2, solution as eluant. Using an electrochemical detector set at + 0.40 volts vs Ag/AgCl electrode, sulfites equivalent to 0.1 ppm SO2 in the extract could be detected with detector response linear up to 6 ppm SO2.
The Most Important Questions about Sulfite Ion You Can’t Miss
How many electrons does a sulfide ion have?
There are 18 electrons for sulfide ion and 16 electrons for neutral sulfur.
You can learn how to find electrons and protons for the Sulfide ion (S 2-) by the following video:
Is sulfite ion polar or nonpolar?
Sulfite ion is polar
Note: In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other.
Sulfite ion has a directional dipole moment and there is also a difference of Electronegativity between sulfur and oxygen atom so it is polar.
Is sulfite ionic or covalent?
Sulfite is ionic. In a covalent compound, valence electrons are shared between the two atoms in the bond. These can be evenly shared (covalent bond) or unevenly shared (polar covalent bond). In an ionic bond, electrons are localized to one of the atoms (giving it an overall negative charge), while the other atom has an overall positive charge.
The difference in electronegativity between the two atoms in the bond can help predict whether the bond is likely to be ionic, covalent, or polar covalent, as can the type of atoms involved (metals or non-metals). A bond with two identical atoms is always pure covalent, while a covalent bond with two different atoms is likely to be polar covalent.
Sulfite ion is a polyatomic ion that is composed of two or more atoms that are linked by covalent bonds, but that still have a net deficiency or surplus of electrons, resulting in an overall charge on the group.
How many valence electrons are in the sulfite ion so−23?
A Sulfite molecule is made up of one Sulphur and three oxygen atoms.
Total number of electrons in Sulphur =6
Total number of electrons in oxygen =3(6)=18
Electrons added due to negative charge =2
Hence, sulfites have electrons =6+18+2=26 electrons.
So, SO32- has a total of 26 valence electrons.
Is sulfite a polyatomic ion?
Yes, Sulfite ion is a polyatomic ion that is composed of two or more atoms that are linked by covalent bonds, but that still have a net deficiency or surplus of electrons, resulting in an overall charge on the group.
What is sulfite ion oxidation number?
By the rules of assigning oxidation number, the oxidation number of oxygen is -2 in compounds.
The sum of oxidation numbers in a polyatomic ion is equal to the charge of the ion.
So, the oxidation number of sulfite ion is +4.
Oxidation states simplify the process of determining what is being oxidized and what is being reduced in redox reactions.
Do sulfite ions have a dipole?
Yes, sulfite ions have a net dipole moment 2.04D.
Is a sulfite ion an acid or base?
Sulfite ion is a weak base, but it undergoes some hydrolysis to produce basic solutions. In acidic solution, the equilibria are shifted to form sulfurous acid, resulting in the evolution of SO2 gas. Sulfur dioxide is a colorless gas with a characteristic choking odor.
Is sulfite ion aqueous solution?
The sulfites of Na+, K+, and NH+4 are soluble in water. Most other sulfites are insoluble in water. However, due to the basic nature of SO32−, all sulfites dissolve in acidic solution.
Is sulfite ion trigonal planar?
The Lewis diagram shows sulfur at the center with one lone electron pair. The sulfur and one oxygen are bonded through a double bond which counts as “one electron pair”. Hence the molecule has four electron pairs and is tetrahedral (trigonal pyramidal not trigonal planar).
Why is SO3 called sulfite?
SO3 is not called sulfite, as the key difference between sulfite and sulfur trioxide (SO3) is that sulfite is an ionic compound having the sulfate (IV) anion whereas sulfur trioxide is a non-ionic compound. Sulfite and sulfur trioxide are chemical compounds containing sulfur atoms. … Sulfur trioxide is an inorganic compound having the chemical formula SO3.
In SO3 there are three double bonds, and no lone pairs at sulfur. Adding two extra electrons changes that by giving two more electrons to sulfur. SO3 is sulfate trioxide, while SO3 -2 is called the sulfite ion.
PraxiLabs virtual science labs enable you to conduct various laboratory experiments in physics, chemistry, and biology online anytime and anywhere.
Create your free account and try the virtual labs that explain all about sulfite ion.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science






