Last Updated on May 7, 2025 by Muhamed Elmesery
Have you ever thought about how we can get fresh water from sea water? Or how can we get the skim milk? Or how is blood separated into its four components in hospitals and blood banks?
Sand and water/ Cereal and milk/ Sugar and salt/ Salt and water/ Water and ethanol/ Water and pepper/ Cement/ Blood, all of these are examples of mixtures that can be separated by physical methods. We can find many examples of mixtures around us in our daily life.
In this article, we will discuss mixtures and separating mixtures techniques.

Table of Contents
What is Meant by Mixtures?
Mixtures are materials that consist of 2 or more substances which don’t combine or mix together by the chemical methods (no chemical reactions happen between the components). Any matter that is not a mixture is a pure matter.
If you look around, you will find many mixtures everywhere, like the air we breathe, our earth, the ground and the water of oceans, sea and rivers. The air is a mixture of gasses like oxygen, CO2, nitrogen and more. While the water of the sea and oceans are mixtures of salt, natural water and other minerals.
The most distinctive property of mixtures is the ability to separate each component easily by physical methods and without any chemical changes. Each substance or component retains its individual chemical and physical properties without any changes.
So we can say that the process of separating mixtures is just a mechanical separating process as each component does not share any chemical bonds.
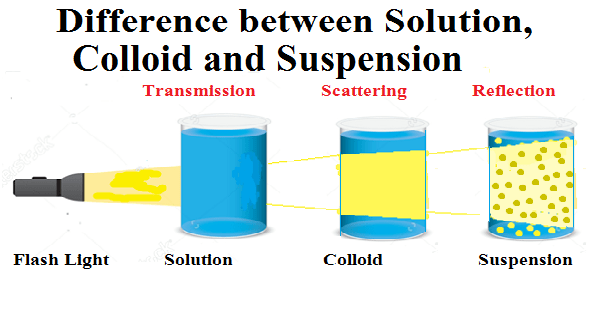
Types of Mixtures in Nature
There are many types of mixtures, and each type has its own unique properties.
-
Colloidal Mixtures
In a colloidal mixture, the particles are so small that they cannot be separated from the solution by filtration or centrifugation. These mixtures typically consist of two or more substances dissolved in a liquid solvent. Some examples of colloidal mixtures are milk, gelatin, paint, and water.
-
Solution Mixtures
In a solution mixture, the substances are not dissolved completely in the liquid solvent. Instead, they form small particles that are suspended in the liquid. As a result, these solutions can be separated by filtration or centrifugation. Examples of solution mixtures are water with sugar and salt, vinegar and oil, and blood and plasma.
-
Suspension Mixtures
In a suspension mixture, the substances are evenly dispersed throughout the liquid solvent. As a result, the suspension can be easily separated by filtration or centrifugation. Examples of suspension mixtures are toothpaste, concrete, and clay.
-
Emulsion Mixtures
In an emulsion mixture, the two substances are evenly distributed throughout the liquid solvent, but the liquids do not mix completely. As a result, the mixture can be separated into two parts by the addition of a small amount of liquid to the solvent. Examples of emulsion mixtures are shampoo and mayonnaise.
Mixtures Characteristics You Can’t miss
Before talking about separating mixtures we want to make sure you know the following mixtures characteristics:
- The components of the mixture which are 2 substances or more and found together can be heterogeneous or homogeneous in nature.
- There is no force acting between the substances of mixture.
- The properties of each component or substance determine the properties of the whole mixture.
- Only physical methods are used for separating mixtures into their dissolved individual substances.
- Mixture properties like melting point and boiling point are determined by the individual substances properties (melting and boiling points).
- Matters or substances in solid or liquid or gas state can combine together and form the mixture.
- During the formation of the mixture, there is no change in the energy.
- Each individual substance of a mixture retains its original chemical and physical properties without any changes.
- Each component of a mixture can be found in any ratio (varies proportions).
Separating Mixtures Examples
Mixtures are easier to be separated than any pure substances. For example, if we have a mixture of some nails have been dropped into a sandpit and they can’t be seen clearly. One methods of separating the nails from this mixture is by using a magnet as it can attract the steel nails. This works because the sand and nails differ in their properties as the steel nails is attracted to the magnets.
Another example for more understanding what is “separating mixtures”:
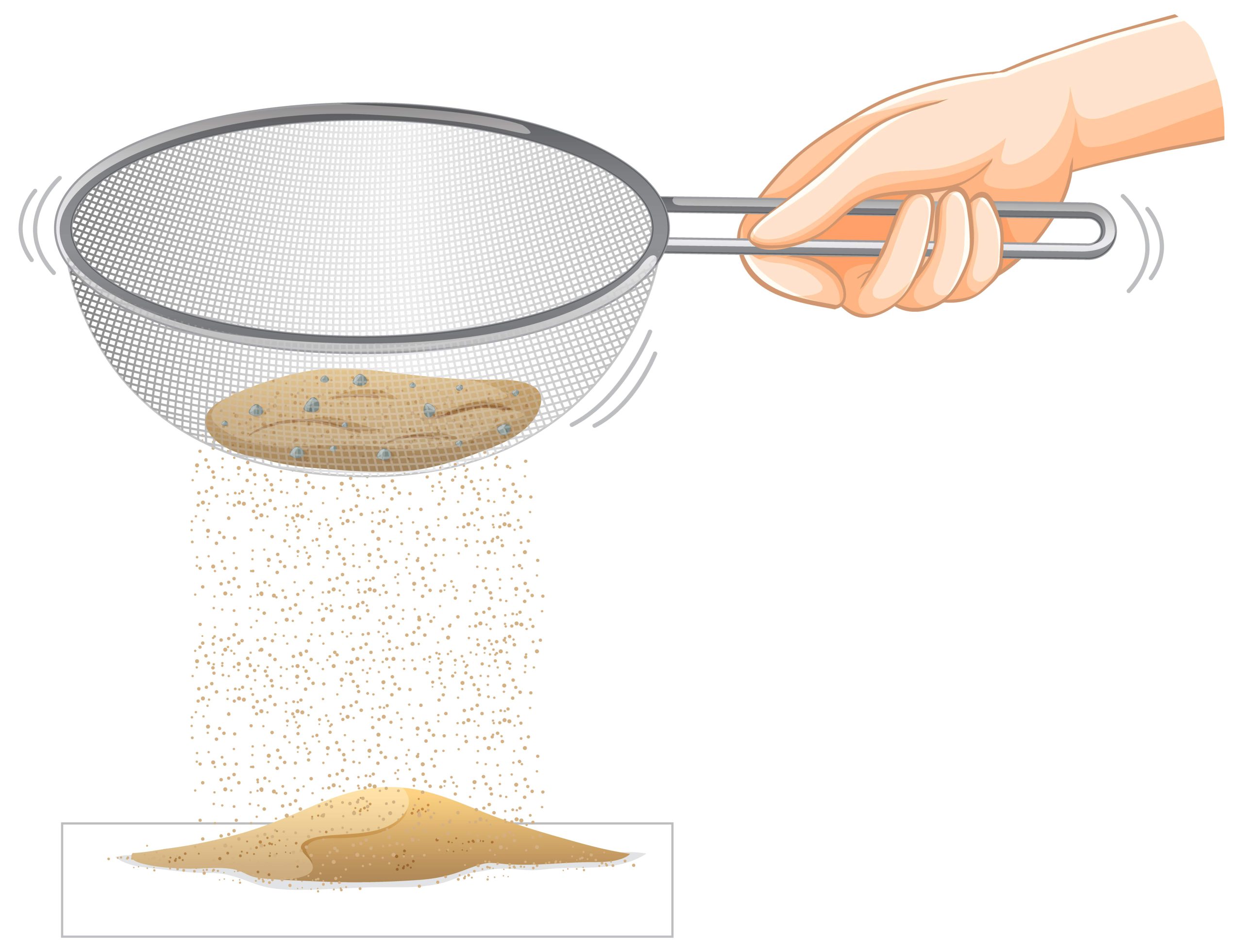
It is not possible to separate the plastic beads and sand with a magnet. The key to separating them is recognizing the different properties of the plastic beads and the sand. An obvious difference is size. The plastic beads are much bigger than grains of sand. A simple sand sieve would do the trick.
Methods of Separating Mixtures
Because any mixture tends to come in different forms, scientists create several methods of separating mixtures that are used to segregate or separate a mixture of substances into its individual components and another important purpose for separating mixtures process is removing the unwanted materials and obtaining only the useful and wanted components.
Mixtures can be separated by using several of the following methods:
- Filtration.
- Distillation.
- Condensation.
- Magnetism.
- Chromatography.
Note: There are many methods of separating mixtures we use in our daily life like handpicking, winnowing, sieving and threshing.
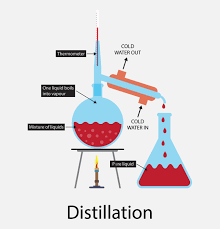
Distillation – Separating Mixtures
Distillation is a separating mixtures technique that is used mainly to extract a mixture of solid found in a liquid. Distillation is suitable in the case of extraction of both liquids and solids from the solution. The simple distillation is the method that is based on the differences between the corresponding vapor pressures and volatility which are found in the components of a mixture.
The most common 4 types of distillation separating mixtures techniques are simple distillation, steam distillation, fractional distillation, and vacuum distillation.
Simple distillation process depends on the following steps:
First, heating the liquid mixture in order to form vapors.
Then, condensing the precious resulting vapors to get back a new liquid. This new liquid which is obtained by the process of condensation is known as distillate.
Note: there are 2 main types of liquids (miscible liquids and immiscible liquids).
In the miscible one, liquids mix together and form a solution (ex: ethanol and water). But in the case of immiscible ones, liquids don’t mix well together like a mix of oil and water.
Distillation method is used for the miscible liquids separation which have sufficient difference in their boiling points and can be boiled without decomposition.
So we can say that distillation is a separation method that is used to extract a mixture of compounds in the solid phase present in a liquid phase. It involves heating of a liquid sample at constant pressure with respect to its boiling point, until all its components are either boiled out of it or condensed.
PraxiLabs is the best platform to simulate 3D science experiments in Physics, Chemistry and Biology.
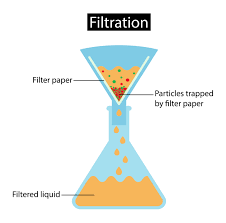
Separating Mixtures – Filtration
Filtration is a separation method used mainly for separating mixtures that contain non dissociated solid in a liquid. It can be also used for removing impurities from the mixture. The filtration method is probably the most common one. It involves using some type of material that separates the various substances. Filtration can be done in many ways depending on the substance you are trying to separate and the material you are using.
Filtration is the most common technique of separating insoluble solid from liquid.
The process of separating mixtures through filtration can be done by many techniques (depending on the purpose of filtration) like simple glass funnel method, Buchner funnel method, or gravity method, or vacuum method.
So we can say that filtration is the process of separating a fluid mixture by flowing it through a filter medium that retains particles smaller than the openings of the filter and this differs from evaporation in that the fluid remains a liquid during filtration.
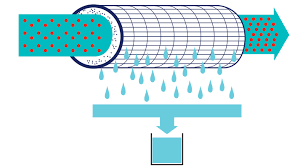
Examples of Filtration in Separating Mixtures
Filtration method enables us to separate liquids of different densities using different filtering mediums. For example, in wastewater treatment, water and organic matter are filtered out by using different filters.
In pharmaceutical manufacturing, oils and solid particles are separated from water-based fluids through the use of a filtration system. Oil and water are both immiscible, meaning they cannot mix freely with each other. This causes oil to sink to the bottom of any container it is placed in, while the water stays at the top. During filtration, the denser oil sinks to the bottom and the lighter water rises to the top. Then, the separation continues through a pipe at the bottom of the container where the water flows out while the oil is retained in the container. This process is called gravity filtration.
An example of filtration in separating mixtures from our daily life is using coffee filters or tea bags. The filtration of mixture made from sand and water is another example of filtration.
Types of Mixtures That Can Be Separated By Filtration
- Solid and liquid mixture.
- Solid and solid mixture.
- Solid and gas mixture.
The following video shows the steps of separating mixtures filtration in detail.
Magnetism – Separating Mixtures
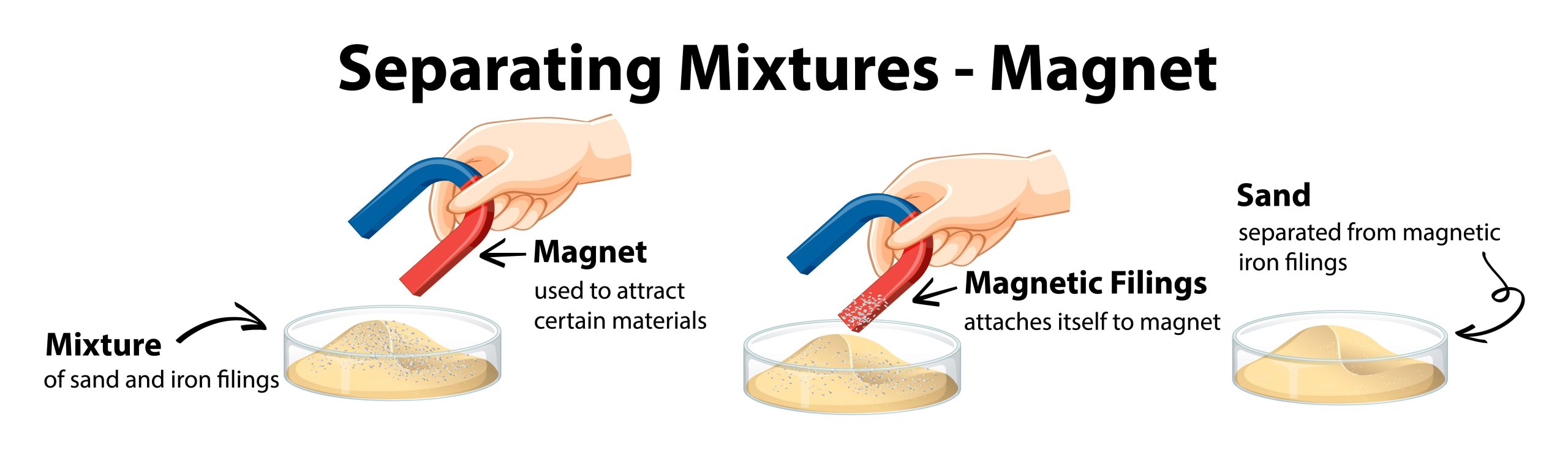
You may be wondering now, how can magnetism be used to separate mixtures?
Magnetism as a separating mixtures technique can be used in case of separating two solids which one of them has magnetic properties. Depending on the difference in magnetic property of the mixture compound the process of separation is done.
In this method, strong magnets are used to separate magnetic substances. The substance with magnetic property attracts to the magnet and separates from the whole mixture.
For example: sulfur and iron mixture.
Magnetism separating mixtures technique is used in the process of iron bearing minerals removal from silica sand for the production of glass.
Chromatography Separating Mixtures
Chromatography separating mixtures is considered as one of the most important and simple techniques used for separating mixtures.
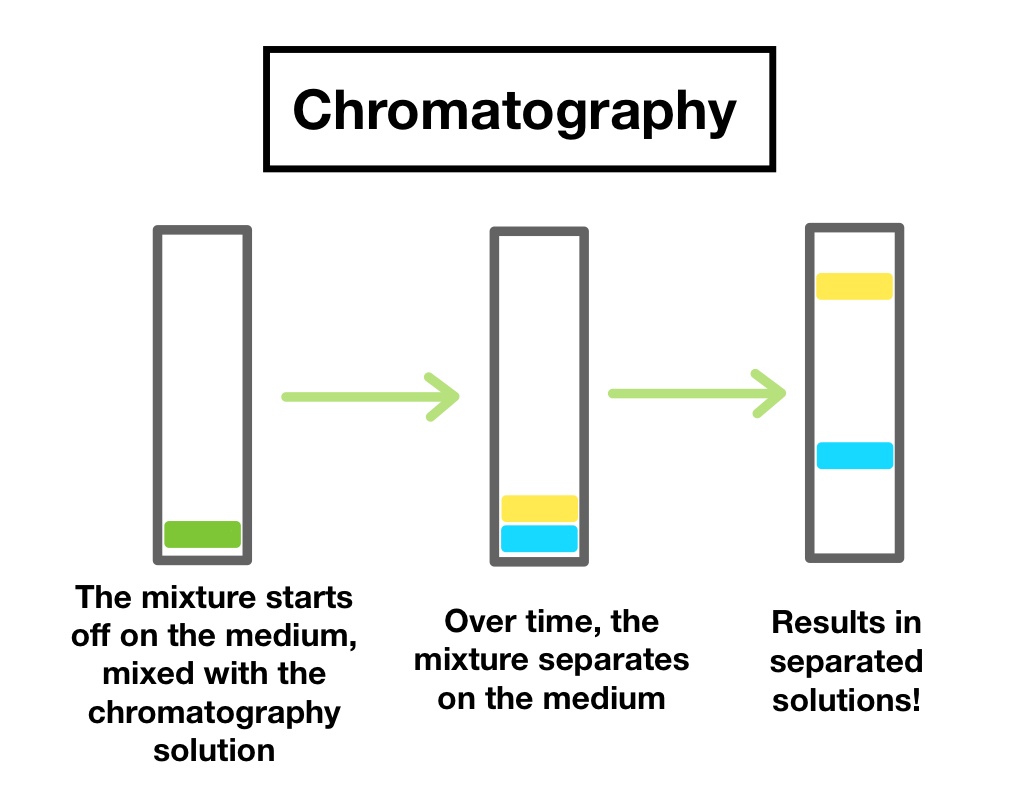
Chromatography separating mixtures method can be used in the following cases:
- Separating mixtures of liquids.
- Separating mixtures of solids.
- Separating mixtures of liquids and solids combined together.
- Separating mixtures of gasses.
The chromatography consists of 2 main elements or phases, the stationary phase and the mobile phase. The stationary phases can be made from alumina or silica gel or paper. The mobile phase is often either a solvent or a mixture of more than one solvent that should be chosen carefully to succeed the process.
Chromatography Separating Mixtures Steps
First, place the mixture you want to separate on the stationary phase.
Then, the mobile phase will pass through the mixture and also through the stationary phase carrying along the constituents of the mixture.
In the case of a constituent with greater affinity for the mobile phase than the stationary one, the constituent will be carried easily with the mobile phase.
In case of a constituent with greater affinity for the stationary phase than the mobile one, the constituent won’t be carried easily with the mobile phase.
The principle of separation here depends on the difference affinity of the mixture constituents for the mobile and stationary phases.
Note: the previous principle is the basic technique for the following types of chromatography: TLC or Thin layer chromatography, Gas chromatography, Column chromatography and High-performance liquid chromatography.

Separating Mixtures Experiment from PraxiLabs
PraxiLabs provides a High-performance liquid chromatography virtual lab which is considered as an example of separating mixtures virtual lab.
A high-performance liquid chromatography (HPLC) system is an analytical method that separates, identifies, and quantifies compounds in mixtures. HPLC uses a high-performance liquid chromatography column to separate samples. The columns contain a stationary phase of stationary particles, usually silica gel particles or a polymeric material coated with silane or some similar chemical. The mobile phase flows through the column at a constant rate and carries sample components away from the injection port and into the column packed with particles. Like all liquid chromatography techniques, HPLC can separate compounds using different types of interactions between analytes and stationary phases.
High-performance liquid chromatography experiment from PraxiLabs is used for separation and detection of amino acids in a tissue.
This separating mixture virtual experiment has been designed to provide a basic understanding of the principle and applications of high performance liquid chromatography (HPLC). The simulation is intended for students with little or no previous knowledge of this technique. The following points will be covered:
- Basic Theory and Principles of HPLC.
- Preparing a Mobile Phase.
- Preparing a Sample Solution for HPLC.
- Using Size Exclusion Columns in HPLC.
- Sample derivatization/ injection by Performing online OPA/FMOC

Separating Mixtures Activity
This is an activity to help students learn how to:
- Explore the different properties of matter than enable mixtures to be separated.
- Demonstrate the separation of mixtures through filtration and evaporation.
Materials needed
- Salt.
- Spoon.
- Coffee filters.
- Water.
- Cups.
- Sand.
- Straw.
Experimental Procedure
- Stir a spoonful of sand into a half a cup of warm water. What happens to the sand? Record your observations.
- In another cup, stir a spoonful of salt into a half a cup of warm water. What happens to the salt? Record your observations.
- Stick the straw into the salt water mixture. Take a small sip. What does it taste like? What does this prove about the salt?
- Look at the sand mixture and the salt mixture. How are the mixtures different? Record your observations. Do you think the mixtures can be separated?
- Place a coffee filter over one of the empty cups. Carefully and slowly pour the sand mixture into the filter. Record your observations. What happens to the water and the sand?
- Try the same filtering method with the sand water. What happened? Taste the “filtered” salt water again with a straw. What do you notice about the taste?
- Pour a small amount of salt water into another cup. Set it on a windowsill and observe it every day for a few days. Record your observations. After the water is gone, what is left behind?
Source: Education.com
PraxiLabs virtual chemistry lab provides more than 50 simulations in chemistry that you can access anytime and anywhere.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





