Last Updated on May 3, 2025 by Muhamed Elmesery
Valence electrons are like the outgoing personalities of atoms. They determine how atoms interact with each other, forming the building blocks of our entire world. Understanding valence electrons is crucial for unlocking the mysteries of chemistry!
In this blog post, we will discover the significance of valence electrons, how to understand valence electrons through virtual labs, and more.
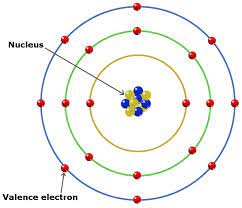
Valence electrons refer to the outermost electrons in an atom (located in the outer shell of the atom) and they describe how stable the atom is. They represent how many electrons are located in an atom’s outermost shell or energy level. They also help in atom interactions and the formation of chemical bonds.
Table of Contents
Valence Electrons Unveiled | The Significance of Valence Electrons
Valence electrons influence various properties of elements:
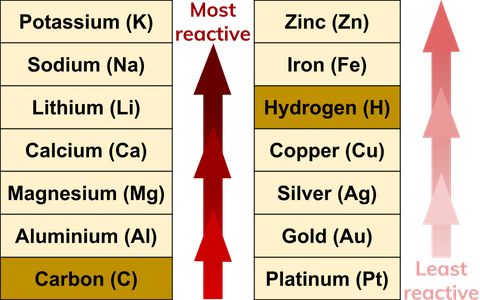
Reactivity
The atom reactivity is mainly determined by valence electrons. Atoms that have a complete shell of valence electrons tend to be chemically inert. Atoms with one or two valence electrons are highly reactive. So, we can say that elements with similar valence electrons tend to react similarly.
Bonding
Valence electrons are the outer shell electrons in an atom and they participate in the formation of chemical bonds (covalently, ionically) through processes of losing, gaining or sharing these electrons. For example, in single covalent bonds, typically, each atom contributes one valence electron to form a shared pair.
Electronegativity
Electronegativity depends on the number of valence electrons. As the number of valence electrons in an atom increases, so does its electronegativity. This increase occurs due to the greater attraction force between the electrons and the nuclei resulting from the increased number of valence electrons. Therefore, electronegativity increases accordingly.
Remember: Electronegativity is influenced by the atomic number of the atom and the distance at which its valence electrons reside from the nucleus. The higher the electronegativity associated with an atom, the greater its ability to attract electrons.
Conductivity
Atom conductivity mainly depends on the number of electrons in its valence shell. An atom with only one electron in the valence shell is almost a perfect conductor, while an atom with eight valence electrons has a complete valence shell and is considered an insulator.
At PraxiLabs you can find different virtual labs simulations in Chemistry anytime and anywhere.
How to Find Valence Electrons Through Virtual Labs
Virtual lab simulations can enrich the experimentation process, guiding students to discover how valence electrons affect the number of bonds an atom can form when creating molecules.
Virtual labs provide a wide range of interactive features:
- Interactive 3D virtual labs provide students with tools that allow for more focus, attention, and engagement. Students stay interested, alert, and absorb information more easily.
- By using virtual labs, you can save time and effort, as they eliminate the need to move between different laboratories. Students can access experiments and learning resources without the need for setup or cleanup, allowing them to focus more on the core learning objectives.
- Virtual labs eliminate potential safety hazards associated with handling hazardous materials or conducting experiments that require specialized equipment. Students can learn and experiment in a safe environment, reducing the chances of accidents or mishaps.
- Virtual labs provide a virtual learning and teaching environment that aims to develop practical skills and learning outcomes.
- Since virtual simulations are accessible via the Internet, students can perform numerous experiments without being confined to specific locations or times, unlike when using real laboratories.
- Students can conduct experiments with 24/7 unlimited accessibility and repeat virtual experiments as many times as needed until they grasp and understand all the information.
Curious About Valence Electrons? Here Are the FAQs You Need
How to find valence electrons?
We can find determine valency using more than one method such as:
1. Using the periodic table for elements (Valence Electrons – Periodic Table)
Scientists use the periodic table to determine the valency of elements by examining their positions on the table. This is based on the principle that all elements in a single vertical column have the same number of valence electrons, and that the atom’s main group number corresponds to the number of valence electrons for those atoms. However, transition metals, located in the rectangle-shaped block made by Groups 3 to 12, are exceptions to this rule.
So, just by checking the number of the group that contains the wanted element, we can determine the valency for the element that is located in that specific column.
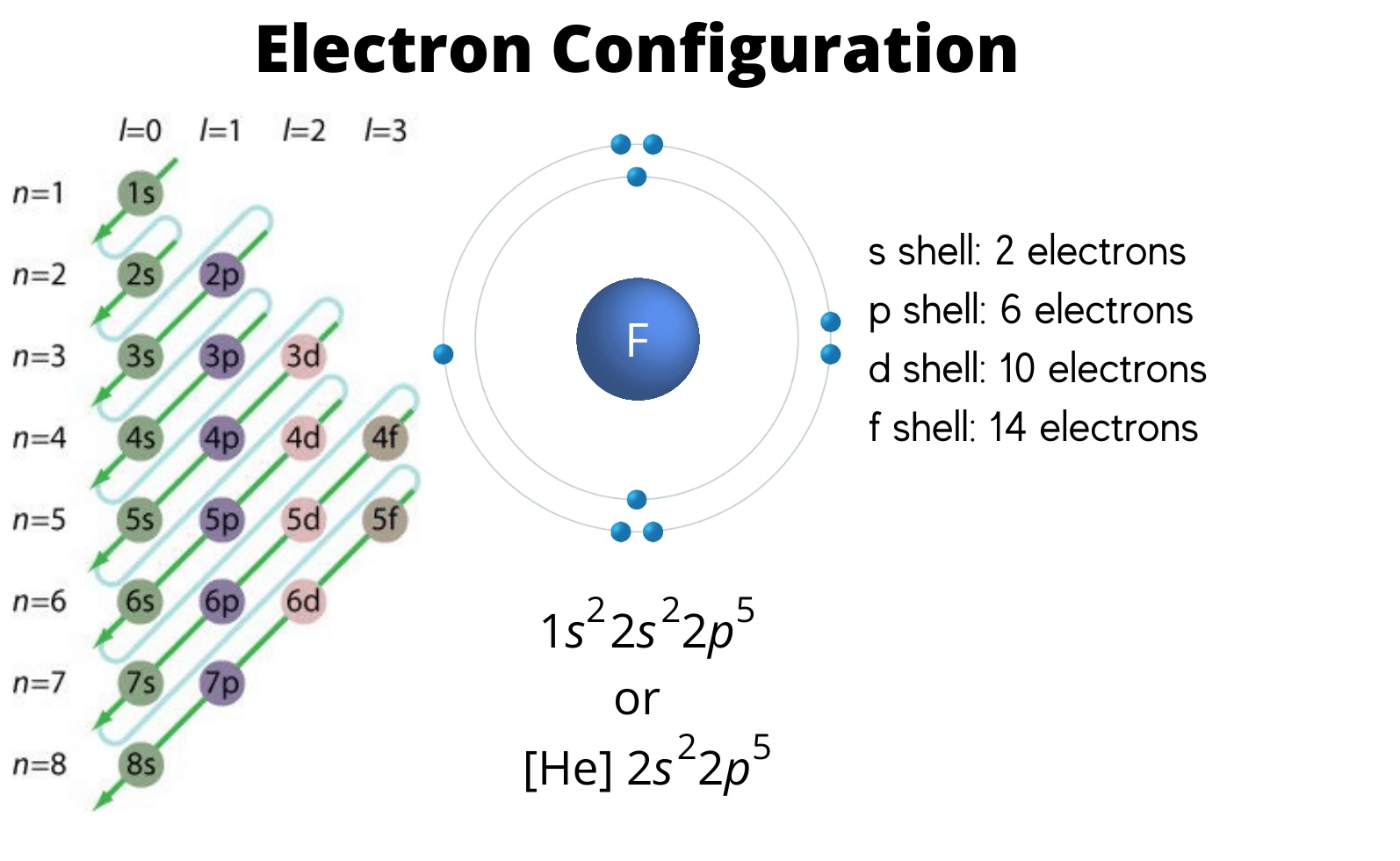
2. Electron configuration
Electron configuration refers to the arrangement of electrons within the orbitals of a molecule or atom, whether atomic or molecular. From the electron configuration, you can determine the number of valence electrons by identifying the electrons in the outermost shell, which is equivalent to the number of valency.
For a better understanding of how to find valence electrons check out our blog
Does Neon have 8 or 0 valence electrons?
Neon has 8 valence electrons, filling its second electron shell and rendering it stable (unreactive) and it doesn’t need to gain electrons from other atoms.
What are the 7 valence electrons?
The family of elements that have 7 electrons in the valence shell is called “halogens”, i.e., fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
What are the 5 valence electrons?
The family of elements with 5 electrons in the valence shell is the elements of Group 15 ”, i.e., nitrogen, phosphorus, arsenic, and bismuth.
Does iodine have 53 electrons?
Yes, because the atomic number of iodine is (53), which means that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus.
How many valence electrons does oxygen have?
By applying the rule that “the number of electrons in the outermost shell is equal to the number of valency”, we find that the number of electrons in the outermost shell “2s and 2p orbitals” is 6. So the number of oxygen valence electrons is 6.
Step into a world of limitless experimentation with PraxiLabs virtual labs in chemistry
Through cutting-edge technology and immersive simulations, PraxiLabs offers a revolutionary approach to learning and exploring the wonders of chemistry. Students can delve into complex chemical reactions, conduct experiments in a safe and controlled environment, and gain practical skills that bridge the gap between theory and application.
At PraxiLabs’ virtual chemistry lab we not only provide a wide range of features, but also focus on making these features count. Who said we couldn’t have both quality and quantity? Our main features stand out with clear differentiators from the competition, in order to provide something for every need.
PraxiLabs provides variety of 3d science experiments in Biology different fields
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





