Last Updated on August 22, 2025 by Muhamed Elmesery
To understand Diels Alder reaction well, we will first clarify important definitions like alkene and diene.
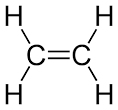
Alkenes
Alkene is a class or a group of hydrocarbons that contains 1 carbon – carbon double bond at least.
Alkene is also known as olefin and its general formula is CnH2n. Alkenes are more reactive than alkanes due to the presence of the carbon double bond that makes alkenes more reactive than alkanes (contain carbon-carbon single bond)
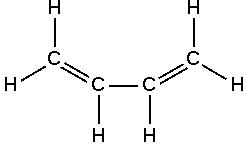
Dienes
Diene is a compound that contains two double bonds, usually between carbon atoms.
There are 3 classes of dienes:
- Cumulated Diene (Allene): this class of dienes contains 2 successive double bonds on adjacent carbon atom.
- Isolated Diene: It contains 2 double bonds separated by 2 or more double bonds.
- Conjugated Diene: It contains 2 conjugated double bonds conjugated or separated by a single bond. It is used widely in polymer industry.
Note: this classification is depends on the position of the double bond in the molecule.
In this article, we will clarify Diels-Alder reaction, its mechanism of action, its examples, its applications, the 3D virtual lab of the experiment that provided by PraxiLabs and more.
In 1928, the German chemists Otto Diels and Kurt Alder discovered Diels-Alder reaction, for which they received the Nobel Prize in Chemistry in 1950.
Try Diels Alder Reaction Experiment Now for Free!
Table of Contents
What Is the Diels Alder Reaction?
In organic chemistry, The Diels-Alder reaction is considered one of the most vital reactions. The reactants of this reaction are a substituted alkene (commonly known as dienophile) and a conjugated diene. Diels Alder reaction gives rise to synthesize six-membered ringed cyclic organic compounds that known as cyclohexene.
So we can define Diels-Alder reaction simply as the reaction between a substituted alkene and a conjugated diene to give a cyclohexene.
Basic Pattern of the Diels-Alder Reaction
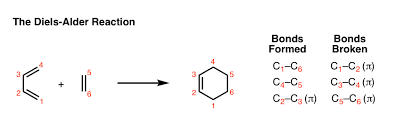
Mechanism of Diels Alder Reaction
Mechanism of Diels Alder reaction depends on the fact that the reaction is a single reaction (pericyclic reactions) occurring between a diene molecule and a dienophile molecule. This reaction generally involves overlapping of highest occupied molecular orbital (HOMO) containing 4π electrons of the diene molecule with the lowest unoccupied molecular orbital (LUMO) containing 2π electrons of dienophile molecule to form a cyclo-addition product, so this reaction is also known as [4+2] cyclo-addition reactions, even though there are many other variations are known now like [2+2] cyclo- addition reactions etc. The driving force of the reaction is the formation of new σ -bonds, which are energetically more stable than the π -bonds.
The reaction occurs in 2 steps:
- The diene reacts with the alkene forming a new cyclohexene ring.
- The new cyclohexene ring rearranges forming the final product.
Diels Alder Reaction Examples
- A common example of Diels Alder reaction is the process of cycloaddition of anthracene ( act as conjugated diene) and maleic anhydride (act as dienophile) to form the compound 9,10-dihydroanthracene-9,10-endo-a3-succinic anhydride. In this reaction, Xylene comound is used as the solvent because of its high-boiling point that could accommodate the high temperature needed to run the chemical reaction.
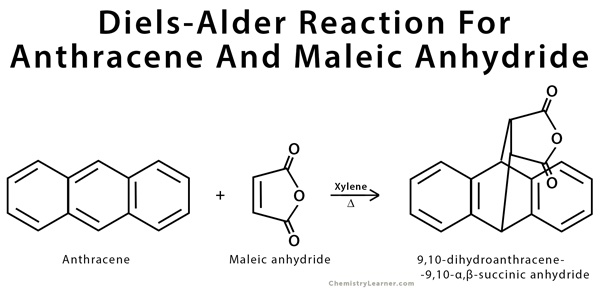
Maleic anhydride is considered as a highly reactive dienophile because there are two electrons withdrawing carbonyl substituents within the chemical structure. In contrast, anthracene compound is not a highly reactive diene because of 2 reasons: its high stability (because of aromaticity.) and its steric hindrance.
- The reaction between 1,3‐butadiene and maleic anhydride.
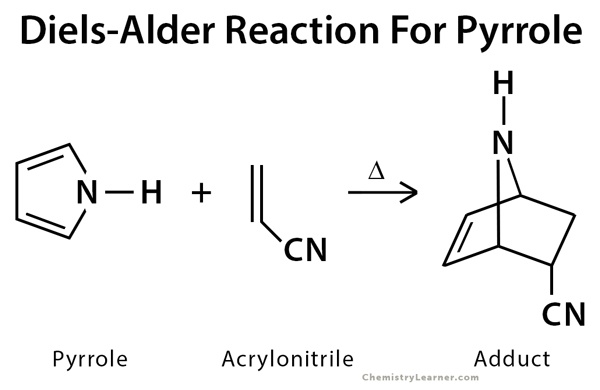
- The reaction between pyrrole and acrylonitrile.
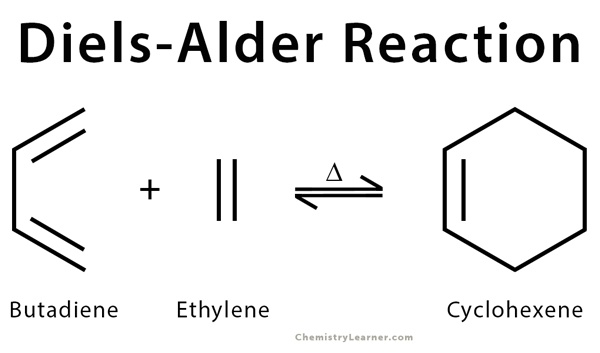
- The reaction between butadiene and ethylene
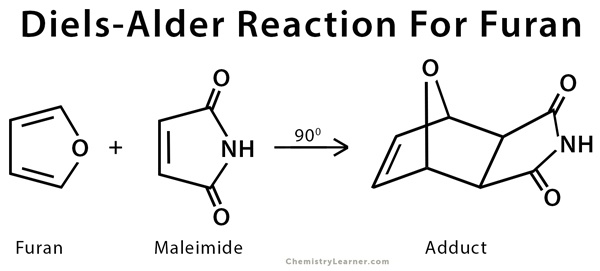
- The reaction between furan and maleimide.
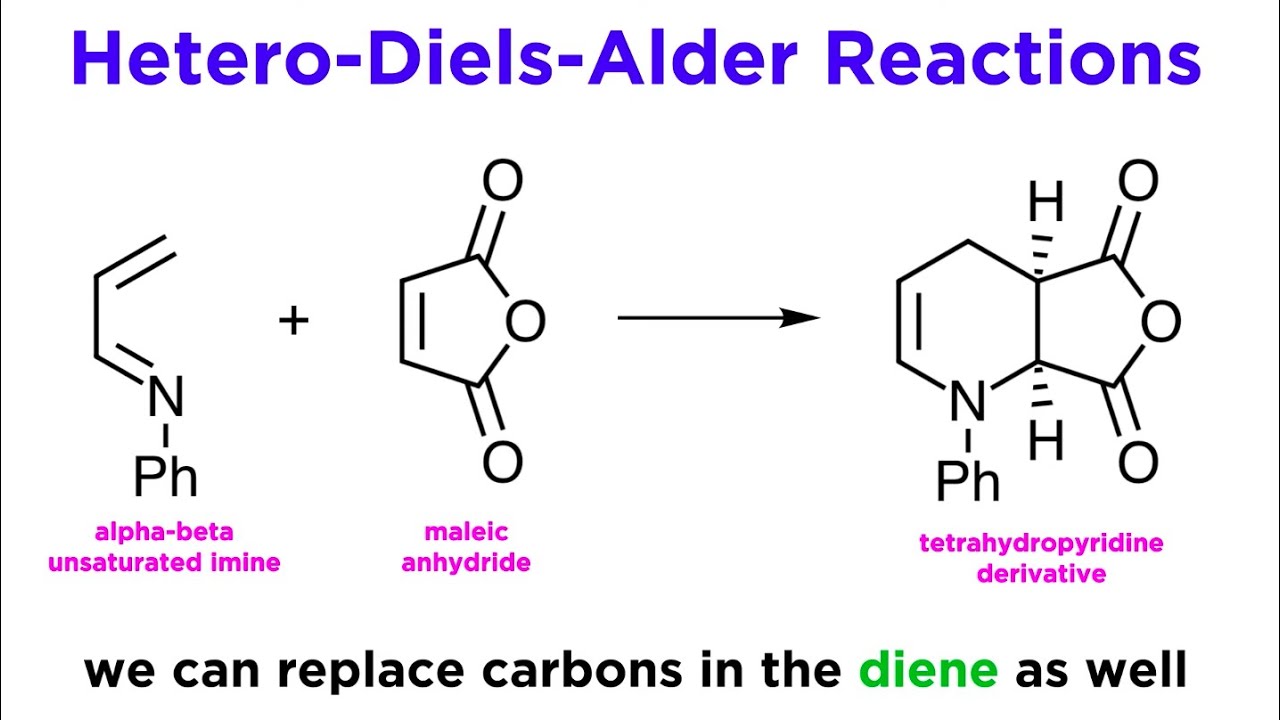
Hetero-Diels-Alder Reaction
The Diels-Alder reaction has many variations. One of these variations is hetero-diels-alder reaction.
In chemistry, hetero refers to any atom that is not hydrogen or carbon.
In hetero-Diels-Alder reaction, either the diene or the alkene (dienophile) contains a heteroatom, most often nitrogen or oxygen. The asymmetric hetero-Diels–Alder reaction of the compounds of carbonyl is considered one of the most powerful methods for the construction of optically active heterocycles, and has been widely used in the process of bioactive natural and synthetic compounds synthesis.
Example of Hetero-Diels-Alder Reaction:
Diels-Alder Reaction Stereochemistry
The method of ‘cube’ is a good method to visualise the relative stereochemistry of Diels- Alder reaction.
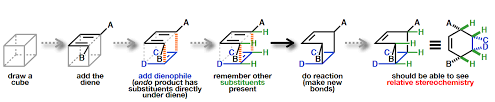
As shown in the previous figure:
- First, draw a cube.
- Then, add the diene.
- Then, add the dienophile.
- Then, remember other substituents present.
- After that, do the reaction and make new bonds.
- Now, you should be able to see the relative stereochemistry of the reaction.
The stereochemistry of the dienophile invariably reacts from the less hindered face and it is preserved in the Diels-Alder product
The “outside” groups on the diene that called “outside” end up on the same face of the new six-membered ring, as do the “inside” groups. When both the diene and dienophile are substituted, diastereomers may form, which we call “exo” and “endo”.
The following video explain Diels-Alder reaction stereochemistry:
Try PraxiLabs Virtual Chemistry Lab For Free and Enjoy Science Education Anywhere and Anytime
Retro-Diels-Alder Reaction
The retro Diels-Alder reaction is the reverse of the Diels-Alder reaction. It passes through the same state of transition when the heat is applied.
In many cases, where the reverse or retro Diels-Alder reaction is exactly the same as the main forward Diels-Alder reaction, all that really happens is that an equilibrium is established between the dienophile/diene and the product of Diels-Alder reaction.
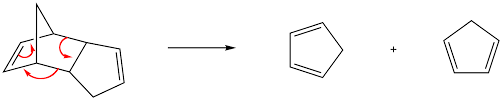
Examples:
Cyclohexene is broken down into butadiene + ethylene (at 800 °C).
Applications of Diels Alder Reaction
Diels Alder Reaction is very important reaction in organic chemistry and has many applications like:
- Natural materials synthesis like plastics and rubber and plastic is one of the most important applications of Diels Alder Reaction
- In the production of vitamin B6.
- In pharmaceuticals and steroids synthesis (ex: cortisone and Vitamin D).
- Preparation of cortisone and cholesterol.
- The synthesis of prostaglandins F2α and E2.
- The synthesis of natural and unnatural poly heterocycles and poly carbocycles.
- The synthesis of dendrimers, and polymers and the preparation of hydrogels for drug delivery.
- Also used in nanotechnology, ex: in the development of nanomedicine that display a preference for efficient, very fast and clean “click” chemistry reactions which allow the active ingredients delivery and subsequent release upon the elevation of temperature.
- Diels-Alder reaction plays a dynamic and vital role in the process of nanomaterials synthesis such as cell-adhesive peptides, block-polymers, dendrimers, crosslinked hydrogels, and drug -delivery systems.

Diels-Alder Reaction Virtual Lab from PraxiLabs
PraxiLabs provides Diels-Alder Reaction Lab Simulation that you can access anytime and anywhere to perform it.
The general aim of the experiment of Diels-Alder Reaction is the synthesis of six member cyclic organic compounds.
Diels-Alder Reaction Principle of Work
One of common diels alder reaction examples is the process of cycloaddition of anthracene ( act as conjugated diene) and maleic anhydride (act as dienophile) to form the compound 9,10-dihydroanthracene-9,10-endo-a3-succinic anhydride. In this reaction, Xylene comound is used as the solvent because of its high-boiling point that could accommodate the high temperature needed to run the chemical reaction.
Maleic anhydride is considered as a highly reactive dienophile because there are two electrons withdrawing carbonyl substituents within the chemical structure. In contrast, anthracene compound is not a highly reactive diene because of 2 reasons: its high stability (because of aromaticity.) and its steric hindrance.
Diels Alder Reaction Method
The method here depend on running an electrocyclic reaction where 4 π-electrons from (anthracene) the conjugated diene and 2 π-electrons from (maleic anhydride) the dienophile are involved in the reaction in the presence of xylene compound as a solvent at temperature of 350 ⁰C. The formation of new σ-bonds is the driving force here because the σ-bonds are energetically more stable than the π-bonds.
By the end of the Diels Alder reaction experiment:
- Students will become proficient at conducting organic chemistry reactions.
- Students will understand the mechanism, principle and procedures of Diels Alder reaction.
- Students will understand the basics of organic synthesis procedures.
- Students will learn the function of Diels Alder reaction and also get trained on how reflux and setup of reaction is used.
Pick The Best Virtual Chemistry labs Plan for You
FAQs about Diels-Alder Reaction
What is the product of a Diels-Alder reaction?
Or
What does Diels-Alder form?
The product of a Diels Alder reaction is six-membered ringed cyclic organic compounds that known as cyclohexene.
What are the characteristics of Diels-Alder reaction?
- The reaction of Diels-Alder forms a new six-membered ring.
- On the dienophile, Electron withdrawing groups ease the reaction.
- On the diene, electron donating groups ease the reaction.
- The diene component must be able to arrange an s-cis conformation.
- The reaction is stereospecific with respect to substituent configuration in both the diene and the dienophile.
- Steric hindrance at the sites of bonding sites may inhibit the reaction. And because of steric hindrance, the s-cis conformation is higher in energy than the s-trans conformation.
What is the importance of a Diels-Alder reaction?
The Diels-Alder reaction is very vital and useful for synthetic organic chemistry as the ring-forming reactions are useful in general in organic chemistry and also because the formation of two new stereocenters in many cases.
Diels Alder reaction is inherently stereospecific. A trans dienophile will generate a ring with trans substitution and A cis dienophile will generate a ring with cis substitution.
What happens in a Diels-Alder reaction?
Diels Alder reaction generally involves overlapping of highest occupied molecular orbital (HOMO) containing 4π electrons of the diene molecule with the lowest unoccupied molecular orbital (LUMO) containing 2π electrons of dienophile molecule to form a cyclo-addition product, so this reaction is also known as [4+2] cyclo-addition reactions, even though there are many other variations are known now like [2+2] cyclo- addition reactions etc. The driving force of the reaction is the formation of new σ -bonds, which are energetically more stable than the π -bonds.
What is Diels-Alder reaction give equation?

The Diels-Alder reaction is considered as cycloaddition of a diene and a dienophile (4 pi + 2 pi ) system which forms a more stable substance because the new formed sigma bonds are more stable than the broken pi bonds.
How do you draw Diels-Alder reaction?
The following video show how do you draw Diels-Alder reaction
Why is Diels-Alder syn addition?
This Diels Alder reaction is a syn cycloaddition reaction because the new 2 carbon-carbon sigma bonds are created on the same face of the dienophile or diene.
Why Diels Alder reaction is considered as a [4+2]-cycloaddition reaction?
Because the reaction involves the addition reaction between a substituted alkene (dienophile) and a conjugated diene molecule in order to create a substituted cyclohexene system.
Specifically, the reaction involves 2 π-electrons from the substituted alkene (dienophile) and 4 π-electrons from the conjugated diene. Therefore, it is known as an electrocyclic reaction whose driving force is the formation of new σ-bonds as they are energetically more stable than the π-bonds.
Why is Diels Alder reaction known as a non-polar reactions?
Because there are no charged intermediates are formed during the reaction and also no unpaired electrons are involved in the addition reaction. It is considered as a concerted reaction as many bonds are formed and broken simultaneously during the transition state.
Why is Diels-Alder stereospecific?
Because the substituents which attached to the dienophile and diene retain their stereochemistry throughout the reaction.
Note: stereospecific refers to the reaction in which the stereochemistry of the reactants controls the products of the reaction.
PraxiLabs’ virtual labs provides Diels Alder reaction simulation that you can access anytime and anywhere
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science






