Last Updated on October 26, 2025 by Muhamed Elmesery
Chemistry virtual labs have changed the way we study the subatomic particles of atom (e.g., electrons, neutrons, and protons) by providing an interactive and immersive environment that enhances the students’ learning experience.

In this blog post, we will learn more about the subatomic particles of atom in virtual labs, and discuss some of the top questions about subatomic particles such as what are the 3 subatomic particles that are found? What are subatomic particles, and how are they explained? What are the 3 quarks subatomic particles? and more.
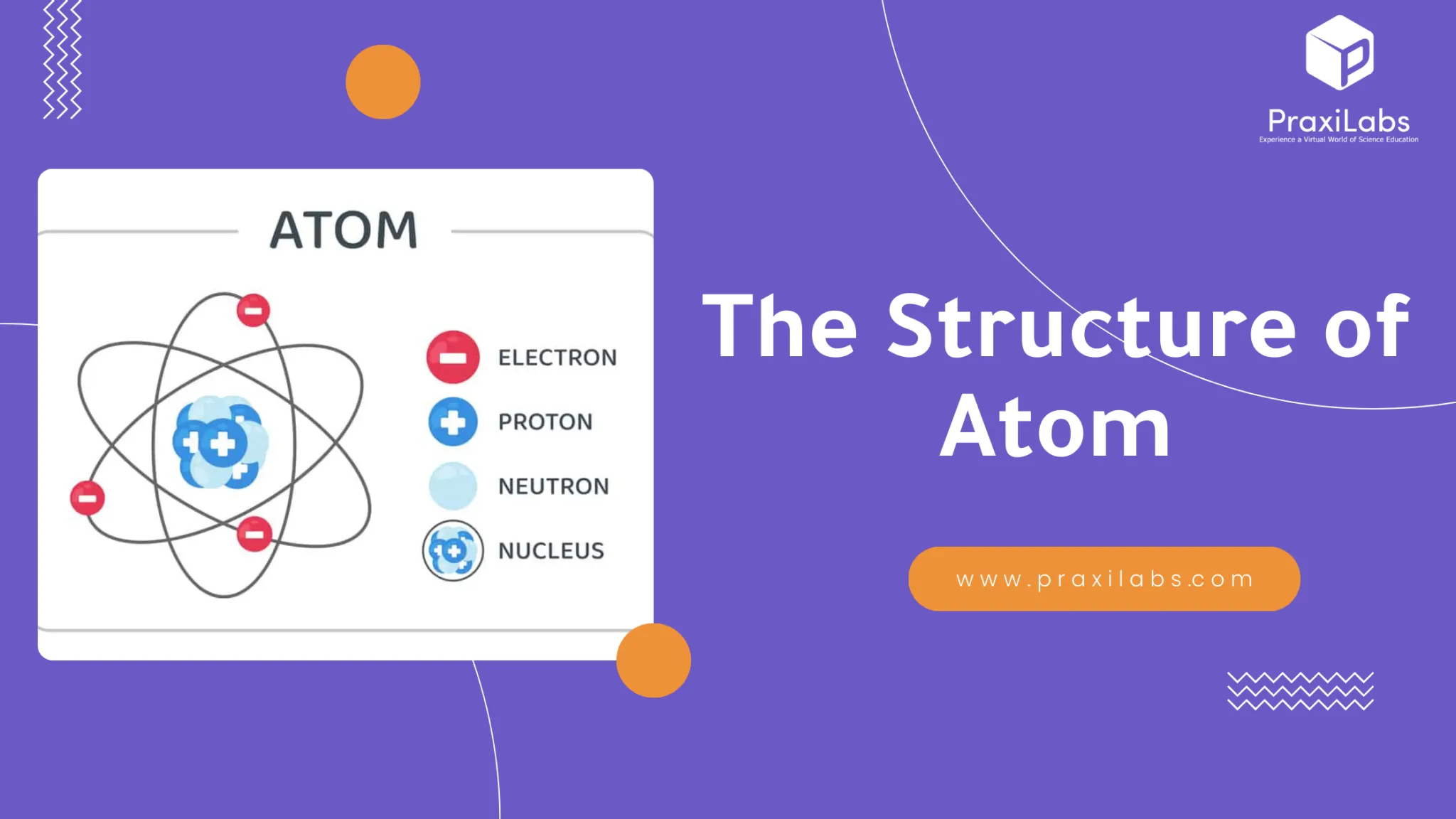
Table of Contents
What are the 3 subatomic particles that are found?
Inside the nucleus of the atom, there are different types of subatomic particles: electrons, protons and neutrons.
| Electrons | Protons | Neutrons | |
| Key Features |
|
|
|
| Location | Outside the nucleus | Inside the nucleus | Inside the nucleus |
| Mass | A negligible mass of 0005 amu | 1.0073 amu | 1.0087 amu |
| Charge | have a negative charge | have a positive charge | Don’t have a charge |
| Discovery | The British physicist Thomson discovered the electron in1897, originally known as “Corpuscles”. | Rutherford established that the nucleus of the hydrogen atom was a positively charged particle, for which he coined the name proton in 1920. | Rutherford predicted it theoretically in 1920 and The British physicist Sir James Chadwick discovered neutrons in 1932. |
PraxiLabs Virtual Labs include a range of online 3D science experiments in physics, chemistry and biology experiments
Pick the Best Virtual Plan or You
What was the second subatomic particle discovered?
Protons were the second subatomic particle discovered after electrons. They were discovered by Ernest Rutherford using cathode ray tubes in 1911. They are positively charged particles that reside inside the nucleus.
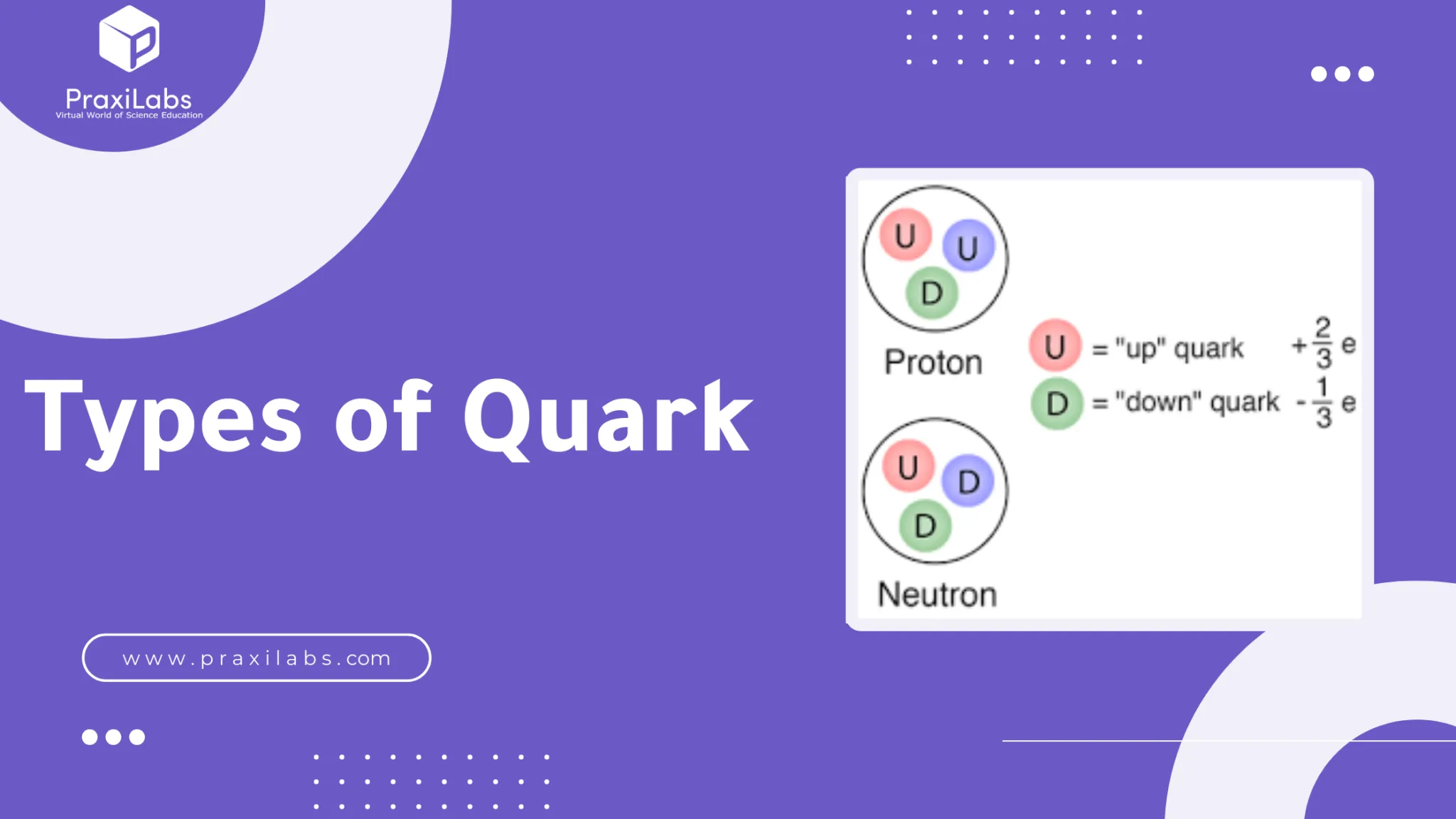
What are the 3 quarks subatomic particles?
A quark is any member of a group of elementary subatomic particles that interact by means of the strong force and are believed to be among the fundamental constituents of matter. Quarks associate with one another via the strong force to make up protons and neutrons, in much the same way that the latter particles combine in various proportions to make up atomic nuclei.
There are six types, or flavors, of quarks that differ from one another in their mass and charge characteristics. These six quark flavors can be grouped in three pairs:
- Up and down.
- Charm and strange.
- Top and bottom.
Quarks appear to be true elementary particles; that is, they have no apparent structure and cannot be resolved into something smaller.

Subatomic Particles of Atom in Virtual Labs
Chemistry virtual lab simulations provide an interactive and immersive method to explore the structure and properties of subatomic particles.
By using 3D atom simulation or subatomic particles of atom in virtual labs, your students will be able to:
- Understand the concept, properties, characteristics of different subatomic particles.
- Detect the number of protons, neutrons and electrons in atoms and ions.
- Define the critical terms related to subatomic particles such as atomic number, mass number, isotopes, and more.
Study | STEM education with atomic structure virtual labs for learners with special education needs
A study presented results from a small-scale evaluation pilot of the Atomic Structure virtual lab with secondary school students who have special education needs. Developed as part of the NEWTON H2020 Project, Atomic Structure virtual labs place the learner in the centre of the learning experience through implementation of personalization, inquiry-based learning, and self-directed learning.
Results analysis of the pictorial Torrance Tests of Creative Thinking (TTCT) showed that the students’ creative thinking has improved significantly in terms of various mental characteristics such as fluidity, flexibility, and originality
Try PraxiLabs Chemistry Virtual Labs
With PraxiLabs virtual labs your students get a full experience, with guidance and learning materials to further aid the learning process rather than simply following through and covering it. With PraxiLabs online labs Your students can actively learn while performing their experiments.
65% of learners are VISUAL.
PraxiLabs provides various multimedia forms
30% are AUDITORY.
PraxiLabs uses VO guidance.
5% are KINAESTHETIC.
PraxiLabs provides more virtual “hands-on” experience.
Additionally, PraxiLabs was designed to fit all learning styles, Each student absorbs knowledge and information differently. While class lectures, textbooks, and lab experiments are each separately effective for different learning style, PraxiLabs combines its 3D virtual lab with additional multimedia and text to provide each learner with material that fits his/her learning style.
Increase your Students’ Learning Retention and Engagement!
Discover Our New Release Experiments in Chemistry
Organic Chemistry
Grignrad Preparation
By the end of Grignrad preparation, your students will be able to:
- Become proficient at running organic chemical reactions.
- Learn the basics of Grignard preparation.
- Understand the mechanism of Grignard reactions.
- Learn the function of Grignard reactions.
- Get trained on how the setup of reaction is used.
Preparation of Ethyl Propionate (Propanoic acid ethyl ester) (Fisher Esterification)
Preparation of Ethyl Propionate (Propanoic acid ethyl ester) (Fisher Esterification) simulation will help your students to:
- Become proficient at running organic chemical reactions.
- Learn the basics of organic synthesis procedures.
- Understand the mechanism of the Fischer Esterification reaction.
- Learn the function of the Fischer Esterification reaction.
- Get trained on how reflux and the reaction setup are used.
Nitration of Methylbenzoate
By the end of Nitration of Methylbenzoate simulation, your students will be able to:
- Become proficient at running organic chemical reactions
- Learn the basics of organic synthesis procedures.
- Understand the mechanism of electrophilic nitration reactions for aromatic rings.
- Learn the function of Electrophilic substitution reactions.
- Get trained on synthetic organic chemistry techniques and skills.
Basic Radical Tests
- Test for Unknown Basic Radical 13
- Test for Unknown Basic Radical 14
In our basic radical tests, students will understand various tests to identify the cation present in a given salt, and the chemical reactions that take place during each test. They will also acquire the skill to perform the experiment in a real lab once they understand the different steps in the procedure.
Visit Our Ever-Expanding Catalog of 3D Chemistry lab Simulations and Enhance Your Students’ Learning Outcomes!
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science