Last Updated on April 15, 2024 by Nourhan Essam
The world is still full of mysteries that we have not yet discovered, and scientists are still trying to understand the nature of this wondrous universe around us. From the structure of the atoms to the details of the supergalactic coordinate systems, our human mind is still incapable of understanding many of these secrets.
In this article, we shed light on the enchanting aspect of our universe. It is an invisible world, yet we can observe its effect in everything around us in our daily life as it is the basic structure of the universe (types of subatomic particles). It is the world of subatomic particles.
We will sail together in this article to explore this world and some of its secrets: what are the types of subatomic particles and how can we study them and study their properties?
Create a FREE Virtual Labs Account Now!
Table of Contents
What Are the Particles?
Before we get to know subatomic particles, let’s first get to know the particles in chemistry and physics.
In the physical sciences, a particle is a small localized object to which can be ascribed several physical or chemical properties, such as volume, density, or mass.
The term particle always refers to objects of small sizes, and the most important types of particles are atomic and subatomic particles, each of which has different properties.
What are Subatomic Particles?
Subatomic particles are particles much smaller than atoms, such as protons, electrons, and neutrons that make up the atom. Protons and neutrons are made up of smaller particles called quarks. Electrons are made up of leptons. Other particles are produced from nuclear reactions, but they are unstable. Let’s start our journey from the atom to understand what it means.
Atom
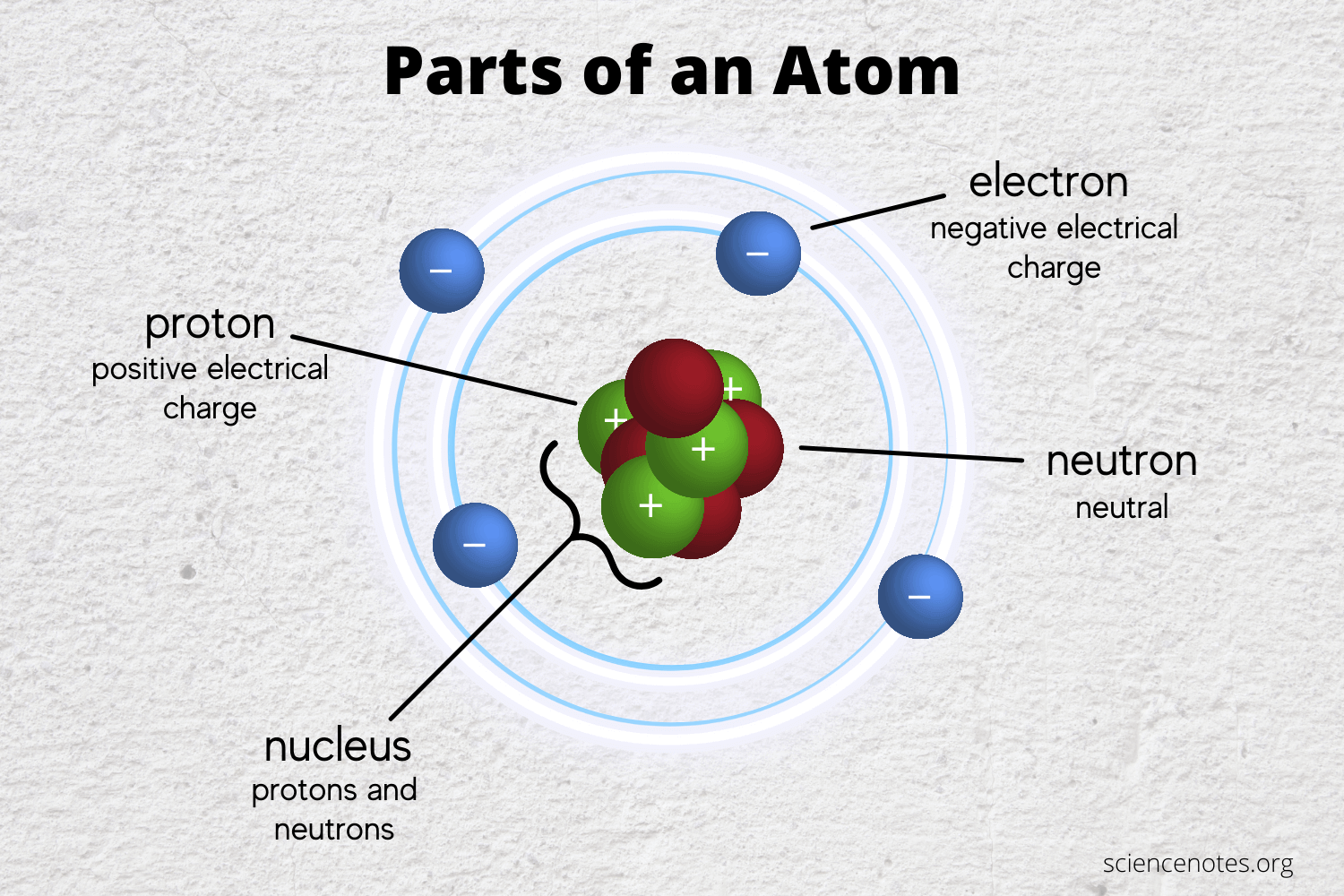
The atom is the smallest building stone or the smallest part of a chemical element that retains the chemical properties of that element, and thus it is considered as the basic unit of matter that controls the structure of the elements. The term “atom” comes from the Greek word Atomus, which means “indivisible.” At that time, the Greeks believed that the atom was the smallest thing in the universe and could not be divided. But now we know that atoms are made up of three types of particles: protons, neutrons, and electrons. We also know that they are made up of smaller particles such as quarks and leptons.
After the Big Bang, 13.7 billion years ago, our very hot universe began to expand and become cooler, allowing the formation of quarks and leptons. Over time, quarks and leptons began to form protons, neutrons and electrons. All of this happened during the first few minutes of the formation of the universe according to “CERN”.
380,000 years after the Big Bang, the temperature was adequate to slow the movement of electrons sufficiently to allow the nuclei to pick them up to form the first atoms in our universe – hydrogen and helium atoms – then, the forces of gravity were now able to perform their magic.
Because of gravity, the withdrawal of gas began to intensify (nebulas), which turned into giant factories of stars. These stars began to form heavy elements, then these elements had scattered throughout the universe because of the explosion of the stars at the end of their lives (supernovas). The process of the birth of stars, the composition of elements, the explosion of stars, and spread of elements in the universe still continue to this moment.
Structure of the nucleus
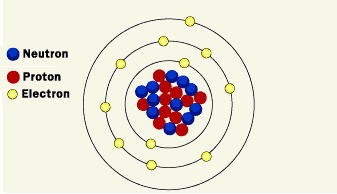
Rutherford was the first one to discover the nucleus more than 100 years ago. He defined the positive particles inside the nucleus and called them protons, and he theoretically predicted the existence of particles with no electric charge, which was confirmed by his student James Chadwick in 1932 when he discovered neutrons. The nucleus represents approximately the entire mass of the atom, where the mass of electrons is very small.
Strong nuclear power (one of the four fundamental forces in nature) allows the components of the nucleus to hold together. This allows the protons to overcome the power of electrical repulsion, so they would not alienate from each other even though they have the same charge.
Visit Our Virtual labs and Experience a Virtual World of Science Education
Try PraxiLabs Virtual Lab For FREE!
Subatomic particles
Inside the nucleus of the atom, we find different types of subatomic particles like protons and neutrons, which are heavier than electrons that rotate around the nucleus in a cloud that is 20,000 times the diameter of the nucleus. The number of protons is always the same as the number of neutrons inside a nucleus. If a neutron is added to the nucleus, it will produce an isotope of the same element, but if a proton is added to the nucleus (what happens inside the stars) it will be a completely new element.
What are The Three Types of Subatomic Particles?
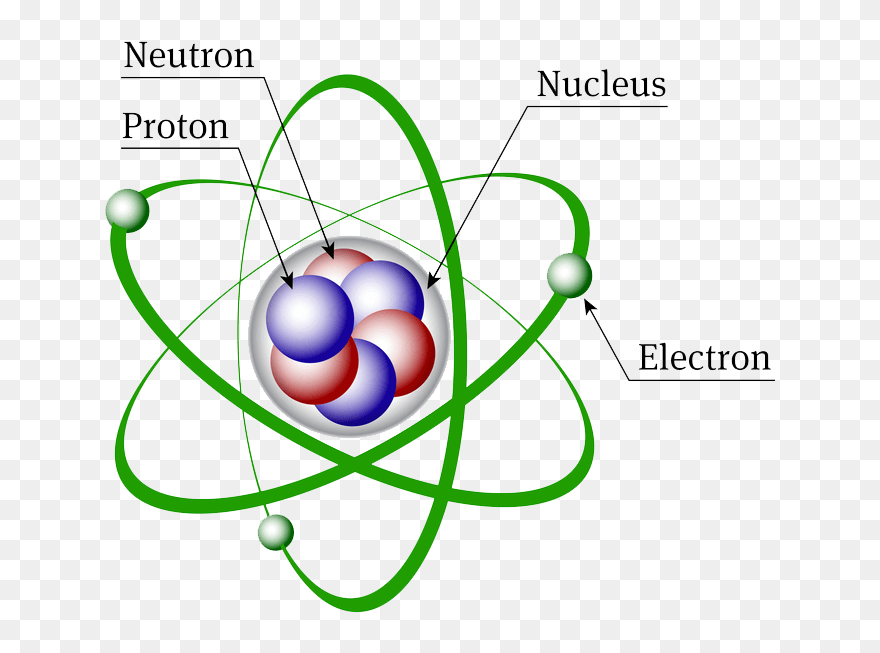
A typical atom consists of 3 types of subatomic particles: protons, neutrons, and electrons
Protons
Rutherford discovered them using cathode ray tubes in 1911, which are positively charged particles that settle inside the nucleus. Their number within the nucleus determines the type of element and also determines the chemical behavior of the element. The number of protons represents the atomic number of the element’s atom, and the element takes its place in the periodic table according to this number.
Protons (one of the types of subatomic particles )consist of smaller particles, the quarks, and each proton consists of 3 quarks, which are grouped together by gluons, which are also subatomic particles but are massless.
Video takes us into virtual travel to the world of atom
Neutrons
Rutherford predicted it theoretically in 1920 and the discovery was practically confirmed by his pupil Chadwick in 1932. Chadwick bombarded a thin sheet of beryllium by alpha particles, a group of particles without charge, which are neutrons.
Neutrons are uncharged particles, having a slightly larger mass than the proton. And, like protons, neutrons are also made up of quarks.
You Can Create a Free Account on the PraxiLabs Online Labs
Get Started Praxilabs For FREE
Electrons
The electron is one of the most important subatomic particle, it is small compared to protons and neutrons. it is 1,800 times smaller than them. If we consider that the neutron mass = 1, the relative mass of the electron is 0.0005439 or about 9.109 × 10-31 kg.
The British physicist Thomson discovered the electron in 1887, originally known as “Corpuscles”. Electrons are negatively charged subatomic particle, and are electrically attracted to positively charged protons. Electrons surround the nucleus in orbits, this idea put forward by the Austrian physicist Schrodinger in the 1920s. This model is known today as a quantum model or an electron cloud model, where the internal orbits near the nucleus are spherical and the external orbits are much more complex.
Electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Using Electron configuration and the principles of physics, chemists can predict the properties of the atom. Such as stability, boiling point, and conduction.
Subatomic Particles Diagram
The following diagram shows the subatomic particles and their charge.
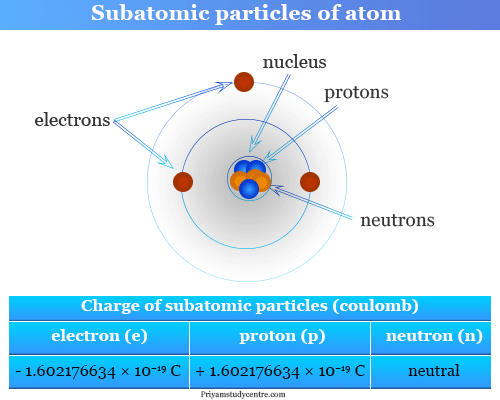
Properties of Subatomic Particles
Now we will discuss the most important properties of subatomic particles:-
- Proton is positively charged particle of the atomic nucleus.
- The atomic number of an element represents the number of protons in the nucleus.
- Electron is negatively charged particle that can occupy a volume of space (orbital) around an atomic nucleus.
- Electrons can be shared or transferred among atoms.
- Neutron is an uncharged particle of the nucleus of all atoms expect hydrogen.
- For a given element, the mass number is the number of protons and neutrons (nucleons) in the nucleus.
The Significance of Subatomic Particles
Subatomic particles have a big importance in various science branches, especially physics and chemistry. Their significance can be understood in the following aspects:
- Fundamental constituents of matter
As it is known, subatomic particles are considered the basic building blocks of matter, forming the fundamental constituents of all matter. They include electrons, neutrons, and protons which are essential for defining the structure and characteristics of atoms.
- Role in atomic structure and interactions
Subatomic particles (electrons, protons, and neutrons) play a vital role in determining the structure and interactions of atoms. They are responsible for the atomic nuclei formation and the arrangement of the electrons in atomic orbitals, which in turn determine the chemical characteristics of elements and their interactions to form molecules.
- Advancements in atomic theory
The discovery and understanding of subatomic particles have led to important advancements in quantum mechanics and atomic theory. These advancements have shown and provided insights into the behavior, characteristics, and interactions of subatomic particles, which contributed to modern physics and chemistry development.
- Particle physics and high-energy physics
The understanding and study of subatomic particles is a fundamental aspect of particle physics and high-energy physics. These fields focus on understanding the behavior, interactions, and characteristics of subatomic particles at both fundamental and high-energy levels, contributing to our understanding of the universe.
- Quantum mechanics and Quantum field theory
The study and understanding of subatomic particles require the application of quantum field theory and quantum mechanics. These theoretical frameworks are essential for analyzing the behavior and processes involving subatomic particles, as well as for understanding the changes in the types and numbers of particles.
The divisible atom
Towards the end of the nineteenth century, it became clear that there was an intimate connection between matter and electricity and that electricity must play some part in chemical change. Gradually it became accepted that atoms existed and that electricity too was’ atomic’ in that it consisted of discrete units of charge.
In the 1890s, Thomson investigated the nature of cathode rays and showed that they consisted of streams of negatively charged particles, i.e. electrons. He estimated the ratio of their charge to their mass, e/m, and he argued that electrons were material parts of atoms, thus initiating the revolutionary idea that atoms were divisible.
Later, Millikan devised a method for finding the charge on an electron and so both charge and mass were known. Working with radioactive elements showed that atoms of heavy elements might disintegrate. Rutherford and Bohr suggested that atoms consisted of a positively charged relatively dense nucleus surrounded by negatively charged electrons.
The number of orbiting electrons gave the atomic number of the element and it was atomic number rather than atomic weight that characterized an element. Elements might consist of two or more atoms with different atomic weights but the same atomic number; such atoms are isotopes.
The Divisible Atom 109 further work showed that electrons were arranged around the nucleus in stable orbits or shells. This could account for the fact that each element showed characteristic spectral lines and could also explain the different types of valency shown by different elements.
Source:
https://link.springer.com/content/pdf/10.1057/9780230597211_6.pdf
Subatomic Particles Virtual Labs | Navigating the Virtual Universe of Subatomic Particles
Chemistry virtual labs play a crucial role in improving the understanding of subatomic particles by providing an interactive and immersive environment that enhances your students’ learning experience. Here are some key reasons why virtual labs are important for understanding subatomic particles:
Interactive Learning Environment:
The 3D interactive virtual lab solution provides students with access to realistic subatomic particle virtual labs and enriches their understanding with a variety of informational and educational content, enhancing their comprehension and learning.
Visualization of abstract concepts
65% of learners are VISUAL, on the other hand, subatomic particles and atomic structure can be abstract and challenging to visualize. So, using virtual labs provides a great chance for students to visualize and interact with these concepts in ways that traditional teaching methods may not allow. Virtual labs provide various multimedia forms.
An enhanced experience with no risks
Virtual labs allow for a unique learning opportunity through making mistakes with no potential safety hazards for the students nor any additional costs due to waste of materials and consumables. Virtual labs provide different scenarios where, if students make a mistake, then just like in the real lab, they will be able to continue, but discover their mistakes when the results come out to be wrong. Furthermore, virtual labs provide a safe environment for experimentation, particularly when dealing with radioactive processes and subatomic interactions.
Enhanced conceptual understanding
Through chemistry virtual labs, students can gain a deeper understanding of the names, properties, interactions of subatomic particles and more. The ability to conduct and interact with the subatomic simulations can enhance the conceptual understanding and retention of knowledge related to subatomic particles.
Subatomic Particles Facts
Which subatomic particles are found in the nucleus of an atom?
Neutron is the heaviest of the three subatomic particles. Neutrons are particles with no charge that have a slightly greater mass than a proton. Like protons, neutrons are also made of quarks.
Which subatomic particle is found outside the nucleus?
The electron is the subatomic particle that is found outside the nucleus.
What are the particles that orbit around the nucleus?
Electrons.
What is the smallest particle of an atom?
The smallest particle in an atom is the quark.
What is smaller than subatomic particles?
The adjective “subatomic” just means “smaller than atoms,” applicable with no lower bound. No matter how small, a thing is “subatomic” as soon as it is smaller than an atom.
A quark is a fundamental particle that is smaller than any measuring instrument we currently have. Following the discovery of quarks inside protons and neutrons in the early 1970s, some theorists suggested quarks might themselves contain particles known as ‘preons’.
The idea wasn’t entirely fanciful, but raises further, as-yet-unanswered questions; for the time being, most physicists believe that quarks, electrons and all other particles are best described as being vibrations of ‘superstrings’, multi-dimensional entities far smaller than the smallest sub-atomic particle.
What are the 3 sub particles of an atom?
A typical atom consists of 3 types of subatomic particles: protons, neutrons, and electrons.
What is the meaning of subatomic particles?
Subatomic particles are smaller than an atom and include particles such as electrons, protons, and neutrons, which are the basic components of atoms. Additionally, there are other elementary particles such as quarks, neutrinos, and positrons, which are also considered subatomic particles.
PraxiLabs Virtual Labs includes a range of online science experiments in physics, chemistry and biology experiments .. Try now!
