Last Updated on October 26, 2025 by Muhamed Elmesery
If you study chemistry, you must have dealt with a substance characterized by its purple color, which is always found in a dark-colored bottle, and it can also stain your hands and clothes..
Yes, it is potassium permanganate, so what is this substance? Its properties? Its uses? And the purpose of its standardization and how? This is what we will discuss in this article and focus on standardization of Potassium Permanganate.

Table of Contents
Properties of Potassium Permanganate
Physical Properties:
| Appearance |
|
| Phase | Solid |
| Odor | No smell but has a sweet taste |
| Boiling point | 100°C |
| Density | 2.703 g/cm³ |
| Solubility |
|
| Solubility in water | 6.38 g/100 ml (20 °C) |
| Melting point | 270 °C decomp |
Chemical Properties:
- Potassium permanganate is an inorganic chemical compound. It is also known as permanganate of potash and Condy’s crystals.
- It’s a strong oxidizing agent with chemical formula KMnO4 , forming a dark brown colored manganese dioxide Mno2 which stains anything that is organic.
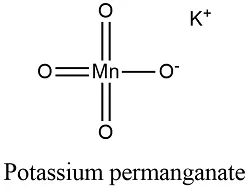
potassium permanganate formula
- The molecular weight/molar mass of KMnO4 is 158.034 g/mol.
- It is produced (in the chemical industry) from manganese dioxide.
- Almost all applications of KMnO4 exploit its oxidizing properties.
- It reacts violently with sulfuric acid resulting in an explosion.
- It reacts immediately with glycerol and simple alcohols producing flames and smoke.
- It acts as a very powerful oxidizing agent in acidic, neutral, and alkaline media.
The equations representing oxidation in these media are as follows:
1) In acidic medium
2KMnO4 +5Na2SO3+ 3H2SO4 → K2SO4 + 2MnSO4 + 5Na2SO4+3H2O
2 KMnO4 + 16 HCL → 2 MnCL2 + 2 KCL + 8 H2O + 5 Cl2
(Concentrated hydrochloric acid makes chlorine)
Note: The manganese containing products from redox reactions depend on the PH. Acidic solutions of permanganate are reduced to the faintly pink Mn2+ ion, as in manganese(II) chloride.
2) In neutral medium
2KMnO4 +3K2SO3+ H2O → 3K2SO3+2MnO2 + 2KOH
In neutral solution, permanganate is reduced to brown manganese(IV) oxide, where Mn is in a +4 oxidation state. Manganese(IV) oxide is the stuff that stains skin when KMnO4 is put on it. KMnO4 spontaneously reduces in a basic solution to green-colored potassium manganate, where manganese is in the +6 oxidation state.
3) In alkaline medium
2KMnO4+Na2SO3+2KOH → 2K2Mno4+Na2SO4+H2O
(MnO42- is reduced)
On heating permanganate crystals decompose to release oxygen.
2KMno4 → K2Mno4 + Mno2 + O2
On diluting permanganate crystals decompose to release oxygen.
2KMno4 + 2H2O Sunlight → 4KOH + 4MnO4+ 3O2

Uses of Potassium Permanganate
- One of the most important industrial applications of KMnO4 is as an oxidizing agent in the chemical synthesis of many important compounds.
- It is used extensively in the water treatment industry. It is used as a regeneration chemical to remove iron and hydrogen sulfide (rotten egg smell) from well water.
- It is used as a disinfectant for cleaning wounds and to cure certain skin conditions like foot fungal infections and dermatitis.
- Another important application of KMnO4 is in the treatment of bacterial infections.
- It is used in printing fabrics and tanning leathers.
- It is used as a bleaching agent, as a pesticide, and as an antiseptic.
- In fuel cell technology, it’s used as an electron receiver in a microbial fuel cell.
- On organic and analytical chemistry, because of its strong color and oxidizing nature, KMnO4 is used in chemistry laboratories as a reagent to calculate the amount of substance that can be oxidized in a sample. In qualitative analysis, this value is referred to as the permanganate value.
The Effect of KMno4 on Health
- In a concentrated form, KMnO4 is an irritant to human eyes and skin. It can react with many reducing agents or organic material because it is inflammable.
- The antibacterial action of KMnO4 is dependent on the process of proteins oxidation of
 bacteria or tissues by this compound. It leaves a stain on skin or tissues.
bacteria or tissues by this compound. It leaves a stain on skin or tissues. - Since it acts by destructive oxidation process on all organic matter, its use is restricted for external purposes only.
- It acts as an antidote in barbiturates, chloral hydrate, and alkaloidal poisoning. A solution of 1:5000 of permanganate, when used as a gastric wash, oxidizes poison and prevents their absorption.
- This compound is usually stored in tightly closed containers. KMnO4 should be handled with care since an explosion may occur when it comes in contact with readily oxidizable substances.
What Happens If Potassium Permanganate Is Consumed?
Ingestion of KMnO4 can cause damage to the upper gastrointestinal tract. Also it may cause systemic toxic effects such as adult respiratory distress syndrome, coagulopathy, hepatic-renal failure, pancreatitis, and even death in severe cases.
Hazards of Potassium Permanganate
- Solid potassium permanganate is a strong oxidizer and in general it should be kept separated from reducing agents.
- Some reactions need a bit of water. For example, powdered KMnO4 and powdered sugar will ignite (but not explode) a few seconds after a drop of water is added.
- Dilute solutions of KMnO4 are not dangerous. KMnO4 forms dangerous products when mixed with concentrated acids.
- KMnO4 stains skin and clothing and should be handled with care. Clothing stains may be washed away using acetic acid. Skin stains disappear within 48 hours.
Visit Our Virtual chemistry labs and Experience a Virtual World of Science Education
Try PraxiLabs Experiments For FREE!
How to Prepare Potassium Permanganate Solution in Lab?
Preparation of Potassium Permanganate – KMnO4
Potassium permanganate is commercially prepared by mixing solution of potassium hydroxide KOH and powdered manganese oxide MnO2, with oxidizing agents like potassium chlorate. The mixture is boiled and evaporates and the residue is heated in iron pans until it has acquired a pasty consistency.
6KOH + 3MnO2 + 6KClO3 → 3K2MnO9 + 6KCl + 3H2O
The potassium manganate (green) formed is boiled with a large quantity of water and a current of chlorine, carbon dioxide CO2, and ozonized air is passed into the liquid until it is converted into permanganate. The MnO2 formed is removed continuously in order to prevent its breaking down.
6K2MnO4 + 3Cl2 → 6KMnO4 (Potassium Permanganate) + 6KCl
The solution of KMnO4 is drawn off from any precipitate of MnO2 concentrated and crystallized. The crystals are centrifuged and dried.
Standardization of Potassium Permanganate –KMno4 with oxalic acid

What Does It Mean by Standardization of Potassium Permanganate? (Aim)
It means to determine the strength of potassium permanganate with a standard solution of oxalic acid. This reaction helps to study the oxidation and reduction theory.
Tools and Reagents for standardization of kmno4:
- Burette.
- Distilled water.
- Pipette.
- Funnel.
- Hot plate.
- 250 ml conical flask.
- 250 ml Beaker.
- Potassium permanganate solution.
- Standard solution of oxalic acid (0.1 M).
- Sulfuric acid.
Procedure ( How to Standardize Potassium Permanganate)
- Clean the burette with distilled water, then empty the water, then rinse the burette with potassium permanganate solution, and then empty the burette.
- Fill the burette with potassium permanganate solution and take the start point.
- Transfer 10 ml of oxalic acid by means of a pipette into clean conical flask; then add 5 ml of dilute sulfuric acid; then heat the solution to 70oC using hot plate in order to fasten the reaction between oxalic acid and potassium permanganate.
- Start titration ( the Ftitration of oxalic acid with kmno4) by gradually adding the potassium permanganate from the burette while continuously shaking the flask till the color changes from purple to colorless.
- Continue adding potassium permanganate until the solution changes from colorless to purple color again and take the end point.
- Record the volume of potassium permanganate used in titration.
- Repeat the previous steps three times; then calculate the average volume of potassium permanganate used in titration; then calculate the concentration of potassium permanganate.
Experimental Data:
| # | Trial 1 | Trial 2 | Trial 3 |
| Initial volume of KMnO4 mL | |||
| Final Volume of KMnO4 mL | |||
| Volume of KMnO4 used mL | |||
| Average volume of KMnO4 mL |
Reaction:
2KMnO4 + 5H2C2O4 + 3H2SO4 → 2MnSO4 + 10CO2 + 18H2O + K2SO4
Calculations:
a2M1V1 = a1M2V2
where
- M1 (concentration of potassium permanganate) = ———— molar
- V1 (average volume of potassium permanganate) = ————mL
- M2 (concentration of oxalic acid) = 0.1 molar
- V2 (volume of oxalic acid) = 10 mL
- a1 (the number of electrons gained per formula unit of potassium permanganate in the balanced chemical equation of half-cell reaction) =2
- a2 (the number of electrons lost per formula unit of oxalic acid in a balanced chemical equation of half-cell reaction) = 5
By substitution in the equation
a2M1V1 = a1M2V2
we get M1= ———–molar
Then by substituting in the previous equation you can calculate the strength of potassium permanganate.
Solution:
Strength = molarity*molecular mass
Molarity=M1=———–molar
Molecular weight of potassium permanganate = 158
Therefore, strength = ———-gram/liter
Conclusion:
From the above experiment, it is evident that potassium permanganate can be effectively standardized by using oxalic acid solution. After performing the calculation, the strength of the prepared potassium permanganate solution was found to be … gram/ liter
Notes:
- The color of potassium permanganate changes with the reaction; no further indicator is used in this experiment to determine the endpoint as the potassium permanganate is a self-indicator.
- We heat the titration flask containing oxalic acid to about 60-70 degrees Celsius and then titrate it against KMnO4. If the temperature is too low (below 55 degrees Celsius), the interaction between the oxalic acid and the potassium permanganate will move too slow. Above 70 degrees Celsius, oxalate acid begins to decompose, so it’s important to stay in this range.
- The reaction between oxalic acid and potassium permanganate is carried out in an acidic medium because permanganate ion in the acidic medium is a very strong oxidizing agent. Acidity is introduced by adding dil. solution of sulfuric acid.
- In this experiment, potassium permanganate is the analyte and oxalic acid is the titrant . Here, potassium permanganate is the oxidizing agent and oxalic acid is the reducing agent.
- Permanganate (MnO4-) ion has a dark purple color. In an acidic medium, MnO4 is reduced to colorless manganous (Mn2+) ions. On reaching the end point, the addition of the last single drop of permanganate imparts a light purple color to the solution.
- Reagents and tools should be handled very carefully.
Why do we standardize potassium permanganate?
The standardization of potassium permanganate used to determine the strength of potassium permanganate with a standard solution of oxalic acid. This reaction helps to study the oxidation and reduction theory.
Try to conduct standardization of potassium permanganate experiment in 3d science simulations for Free
PraxiLabs provides virtual science labs in chemistry for students, teachers, and researchers. Create your free account and enjoy conducting standardization of potassium permanganate experiment online.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science







