Last Updated on May 15, 2025 by Muhamed Elmesery
Chemistry is the branch of science through which interactions and reactions between different atoms, electrons, elements, molecules, and many other particles are being analyzed, tested, and studied.
As a vital branch of science, chemistry exists around us in a daily manner, whereas chemical interactions and reactions do not occur haphazardly; rather they are all governed by many chemistry laws that our scientists managed to figure out many years ago for our human civilization.
And whether you are a science student or not, it is necessary to—at least—be familiar with the most important chemistry laws that govern this science, understand their nature, and maybe study them as essential chemistry knowledge.
In this article, we cover the 9 most critical chemistry laws and how they work… Let’s start!
Get started Praxilabs for FREE
Table of Contents
Chemistry Laws | The Fundamental Chemistry Principles
Chemistry laws are many, and they are subject to changing and evolving with every new detection and discovery. These laws work hand in hand with every other law that we know rules other phenomena and reactions. Whether these laws are biological, mathematical, or physical, they are all still seen as “the laws of nature.”
In chemistry, three laws are looked at as the “fundamental chemistry principles” which govern the behavior of matter.
These 3 basic chemistry concepts are:
- The law of conservation of mass.
- The law of constant proportions.
- The law of multiple proportions.
Throughout your chemistry studies, you may have worked closely with these laws, without necessarily knowing their true nomenclature. Here, we will discuss each one of them in detail.
-
The law of conservation of mass
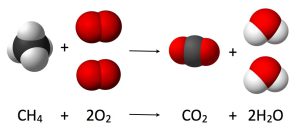
The law of conservation of mass law dates back to Antoine-Laurent de Lavoisier’s discovery in 1789, stating the fact that:
“mass is neither created nor destroyed in chemical reactions.”
In other words, and for a closed system, the total mass of both the reactants and the products of a chemical reaction will remain the same throughout that reaction and at any point in time during that reaction.
This discovery was proven true with calculations and experiments, bearing in mind that almost all naturally formulated elements are very stable given the conditions of our Earth’s surface. By this, the foundation for modern chemistry was laid.
-
The law of constant proportions
Also known as “the law of definite proportions” or “Proust’s law”, it was first stated by the French chemist Louis Proust in 1779, based on his work on sulfides, metallic oxides, and sulfates. Yet, the first observations referring to this law were made by both the chemists Antoine Lavoisier and Joseph Priestley.
The law states that:
“Individual elements constituting a particular chemical compound are present in a fixed ratio (in terms of their mass), regardless of their source.”
For better comprehension, a pure water molecule will always consist of two hydrogen atoms and one oxygen atom, forming the well-known 2:1 ratio. A gram of pure water—in case you are into numbers—approximately includes 0.11 grams of hydrogen and 0.88 grams of oxygen.
Another example of a famous chemical compound obeying this law is methane. It takes four hydrogen atoms and one carbon atom to successfully bring out one methane molecule.
An exception to this law is non-stoichiometric compounds, where the elements’ ratio varies from one sample to another. Also the samples of elements that vary in their isotopic composition and natural polymers are both known to defy and disobey this law.
In a nitrogen dioxide (NO2) molecule, the ratio of the number of nitrogen and oxygen atoms is 1:2, and its mass ratio is 14:32 (or 7:16). Hence, it does not obey the law of constant proportions.
This law was not met by open arms amongst the scientific community in the beginning, rather, with much opposition. It was not until Dalton’s atomic theory that this law was favored. A relation between the theory and the law was established in 1811 by the Swedish chemist Jacob Berzelius, only then was it accepted.
-
The law of multiple proportions
As a consequence of the idea of combining elements to form compounds, introduced by known as “Dalton’s Law,” was proposed in 1804 by the English meteorologist John Dalton. The law states that:
“When elements combine and form compounds, the proportions of those primary elements can be expressed in a ratio of small whole numbers.”
An example is the reaction of carbon and oxygen that can lead to the formation of both carbon monoxide (CO) and carbon dioxide (CO2). In CO, the ratio of the oxygen amount to that of carbon is 1:1, while in CO2, the ratio is 1:2, and both are ratios of simple whole numbers.
This law is one cornerstone of the recognized modern atomic theory and comes side by side with the laws of mass conservation and the law of definite proportions. All together, these laws reinforce Dalton’s assumption and concept which acknowledge matter to be composed of different indivisible combinations of some building blocks of matter, i.e., atoms.
Our current understanding of atomic composition and structure was based on these laws and theories, not to forget including other vital concepts such as molecular, or chemical formulas.
Chemical Formulas
The human’s chemical literacy is incubated by his/her ability to form and include a simple common language which easily expresses and represents a compound, a molecule, or even a single element. All of these come in contact together in order to express a series of reactions and interactions throughout some letters and numbers.
This common language is hence called a chemical formula. And it is defined as follows:
“An expression of chemical symbols and numerical subscripts that represents the composition of one unit of a compound.”
Be careful not to confuse the definition and usage of a “chemical formula” with that of a “structural formula,” where the latter is a 2D graphical representation showing the atoms’ spatial relationships that form the molecule or the compound.
Chemical formulas depend entirely on the compound’s number of consistent atoms and their ratios shown in numbers. These formulas can be simple, i.e., H (referring to hydrogen), or complex, as is the case with CH3CH2OH (referring to ethanol).
It is very necessary for you—if you are a clever student or a scientist—to wholeheartedly know and memorize as many chemical formulas as you can.
Learn More About General Chemistry
General chemistry is a branch of chemistry that focuses on studying matter, energy, and the interactions between them. It serves as a broad introduction to various concepts and basics in chemistry. It is often taught in high school chemistry courses, as well as at the introductory university levels.
General chemistry covers a wide range of topics, including:
- Conservation of mass.
- Elementary atomic theory.
- Periodic table and periodicity.
- Acid-base chemistry.
- Law of constant composition.
- Law of gases.
- Nuclear chemistry.
- Solubility.
- Chemical bonding.
- Chemical kinetics.
- Thermodynamics.
- Electrochemistry.
- Chemical equilibria.
These topics are essential for understanding many aspects of the natural world and various applications of chemistry.
Organic Chemistry Laws
There is a dedicated branch of chemistry that is devotedly concerned with studying and understanding—but not memorizing—organic compounds and all the interactions and relations between them.
Organic chemistry requires passionate students that are truly fond of chemistry, in order to grasp and maintain all its laws and compounds’ nomenclature. This exciting branch is ruled by many of the “regular” chemistry laws, as well as other exclusive ones that are tailored to adhere to its eccentricity.
Aside from learning the basics, organic chemistry can fit well under the umbrella of its “golden rules.” Compost, organic fuel, alcohol, urea, and acetone are forms of organic compounds existing in our day-to-day lives.
5 Live-Changing Laws of Chemistry
Since science is a revolutionizing human-invented tool, it is constantly developing, and its remarkable steps were—and still are—achieved by connecting the dots of all the laws and pieces of information all together, and bingo! A new law, observation, or device is formulated, discovered, or invented.
In this section, 5 gas-related live-changing chemistry laws will be explained and discussed. These laws mainly deal with temperature, pressure, volume, and many other characteristics that control how atoms, elements, and compounds behave in different conditions. The laws are:
-
Avogadro’s Law
If you studied chemistry only as an introductory course, you would have probably dealt with Avogadro’s law. This famous law states that:
“Equal volumes of all gasses, at the same temperature and pressure, have the same number of molecules.”
Hypothesized in 1811, this empirical law was named after Amedeo Avogadro, a higher physics professor at the University of Turin. Nevertheless, his law was not accepted among the scientific society until after 1958, when a logical chemical system, constructed by the Italian chemist Stanislao Cannizzaro, was entirely based on it.

Amedeo proposed that ideal gasses at the same volume, and in the same conditions of temperature and pressure, contain an equal number of molecules! This certain number of molecules is scientifically denoted as Avogadro’s number, and it is equal to 6.02214076 x 1023 molecules.
Its mathematical representation is defined as
V α n, or
V/n = κ
where
V: the volume of the gas
n: the amount of substance of the gas (measured in moles)
k: a constant for a given temperature and pressure
Under two sets of conditions, the relation between same compounds and substances is expressed as
V1/n1=V2/n2.
This equation defines a relation between the number of moles of the gas and the volume of it, where it forms a direct proportion ratio.
-
Boyle’s Law
“At constant temperature, the pressure exerted by a given quantity of gas varies inversely with the volume occupied by it.”
Boyle’s law is another empirical relation that describes and discusses gasses’ compression and expansion. This law was formulated in 1662 by the physicist Robert Boyle, where its mathematical equation is given by
p α 1/V, or
pV = K
where
- P: the pressure exerted by the gas.
V: the volume occupied by the gas.
K: proportionality constant.
If the gas is put in a container and a work is done on it, or by it, the following relation can be used to determine the pressure value:
p1V1 = p2V2.
The law is functionally used in many applications and some of them are mentioned in this interesting article: 3 of the Most Important Applications of Boyle’s Law.”
Boyle’s law practice problems
- A gas occupies 12.3 liters at a pressure of 40.0 mm Hg. What is the volume when the pressure is increased to 60.0 mm Hg?
To solve this problem, we can use Boyle’s law, which states that the pressure and volume of a gas are inversely proportional at a constant temperature.
According to Boyle’s law, we can set up the following equation:
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Plugging in the given values:
(40.0 mm Hg) (12.3 L) = (60.0 mm Hg) (V2)
Simplifying the equation:
492 L mm Hg = 60.0 mm Hg V2
Dividing both sides by 60.0 mm Hg:
V2 = 8.2 L
- A gas occupies 1.56 L at 1.00 atm. What will be the volume of this gas if the pressure increases to 3.00 atm?
To solve this problem, we can use Boyle’s law, which states that the pressure and volume of a gas are inversely proportional at a constant temperature.
According to Boyle’s law, we can set up the following equation:
P1V1 = P2V2
- Plugging in the given values:
(1 atm) (1.56 L) = (3 atm)(V2)
- Simplifying the equation:
1.56 L atm = 3 atm V2
- Dividing both sides by 3 atm:
V2 = 0.52 L
-
Charles’ Law
In his unpublished work from the 1780s, the scientist Jacques Charles formulated this law, which is also known as “the law of volumes”.
The law states that:
“The volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.”
Frankly speaking, this law describes how gasses favor to expand when they are heated.
The mathematical equation is given by
V α T, or
VT = K.
If a comparison is obtained, we can use the following formula:
V1/T1 = V2/T2.
From these equations we deduce that an inverse relation exists between the temperature and the volume.
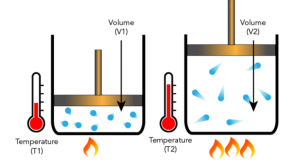
Boyle’s law vs. Charles’s law
Boyle’s law and Charles’s law are two fundamental gas laws that describe the relationship between different properties of gasses. Let’s differentiate between them:
|
Boyle’s Law |
Charles’s Law |
| It states that at a constant temperature, the pressure exerted by a given quantity of gas varies inversely with the volume it occupies. | It states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature if the pressure remains constant. |
| It gives a relation between the volume and the pressure of the gas. | It gives a relation between the volume and the temperature of the gas. |
| Mathematically, Boyle’s law can be expressed as: P1V1 = P2V2
Where: P ——- pressure V ——- volume |
Mathematically, Charles’s law can be expressed as: V1/T1 = V2/T2
Where: V ——— volume T ———-temperature |
| The product of the pressure and the volume is constant (PV = k). | The temperature and volume ratio is constant (V = kT). |
| It is often applied to situations involving changes in the volume of a gas, such as compressing or expanding a gas in a container. | It is often applied to situations involving changes in the volume of a gas due to changes in temperature, such as heating or cooling a gas in a container. |
-
Gay-Lussac’s Law
Formulated in 1808 by the French chemist Joseph Gay-Lussac, this law is another important cornerstone in gas laws. It states that:
“Both the pressure and the temperature of an ideal gas are directly proportional, assuming constant mass and volume.”
The mathematical expression of the law can be written as
P α T, or
P/T = K.
Note that T is the absolute temperature of the gas and not any haphazard temperature.
Gay-Lussac’s effect is tangible in our everyday’s life. Examples can vary from noticing your car tires’ pressure in winter as it drops to keeping an eye on your propane tanks at home. The tires and the propane tanks read higher or lower pressure upon the temperature surrounding them.
-
Ideal Gas Law
It’s also known as “the general gas equation.” And although this law has some limitations, it stands as a good approximation of gasses’ behavior in many different conditions.
The law was formulated in 1834 by Benoît Paul Émile Clapeyron and represents a combination of all the previously mentioned ones, i.e., Avogadro’s, Boyle’s, Charles’s, and Gay-Lussac’s.
The law states that:
“For a given mass and constant volume of an ideal gas, the pressure exerted on the sides of its container is directly proportional to its absolute temperature,”
where this is mathematically expressed as
pV = nRT,
where
- n: the number of the moles of the gas.
R: the ideal gas constant.
Before starting your calculations, make sure that the temperature (T) in use is given in Kelvins. And that R=8.3145 J.K-1.mol-1, or m3⋅Pa⋅K-1⋅mol-1.
All in all, simplicity in this relation encourages us to treat gasses as ideal, unless there is a good reason to do otherwise.
Chemistry, especially organic chemistry, is an ever-evolving science, and it underlies many key laws and rules that steer everything in our lives. Other chemistry laws, such as Faraday’s law, Graham’s law, and Henry’s law, are all pivotal and fit perfectly in their places.
Many names and laws keep appearing when we maneuver from a state to another and from a condition to another. More experiments await you on PraxiLabs —for FREE—if you are seeking more 3D science experiments particularly concerned with chemistry laws or virtual chemistry labs in general.
Sign Up to PraxiLabs and Start Your FREE Virtual Lab Simulation!
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





