Last Updated on August 22, 2025 by Muhamed Elmesery
When you’re reading a scientific text, or having a science lesson regarding bonding and valency, you may come across the term valence electrons. But what does it mean?
This is what we will know in this article in detail. In addition to explaining how to find valence electrons in more than one way and explaining a set of examples for further understanding.
Table of Contents
What are Valence Electrons?
To understand how to find valence electrons well, you should learn first what are valence electrons and the following definitions:
Valence is the number of electrons an atom should gain or lose to reach the nearest inert gas electron configuration (noble gas).
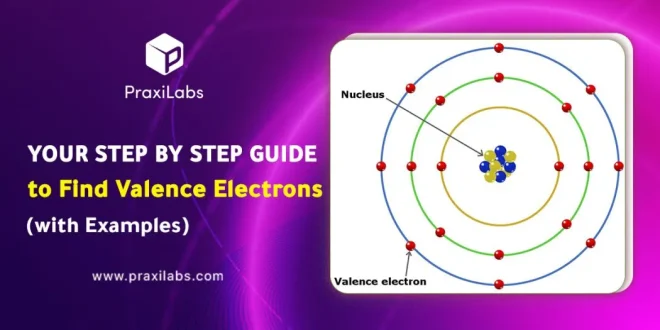
Valence electrons are the outermost electrons in an atom (located in the atom outer shell) and they describe how stable the atom is. Really, valence electrons represent how many electrons are located in an atom’s outermost energy level. They also help in the process of chemical bonds formation and the atom interactions.
We can simply define valency as the electrons (or negatively charged subatomic particles) that are found in the atom outer shells and are not filled.
The number of electrons present in the outermost shell (valence shell) of an atom are called valence electrons.
Valency are an orbital electron present in an atom’s outer shell. Different atoms have different numbers of valence electrons. The inner shells of the atom are usually full and there is usually one or more valence electrons found in an outside shell of an atom.
The number of valence electrons in an atom is typically symbolized by the letter n.
The number of valence electrons in any given atom determines what kind of bonding the atom has with other atoms, whether it’s going to be a metal or nonmetal, and its chemical properties.
The outermost shells of an atom can be occupied or unoccupied. If the shell is unoccupied it is called a valence shell. The number of electrons in a valence shell determines what element that atom is.
Elements differ in how many valence electrons they have, some have as few as one, and some as many as eight.
How to Find Valence Electrons for Atoms?
We can find the valency in more than one method like:
- Using the periodic table for elements (Valence Electrons – Periodic Table)
- Electron Configuration.
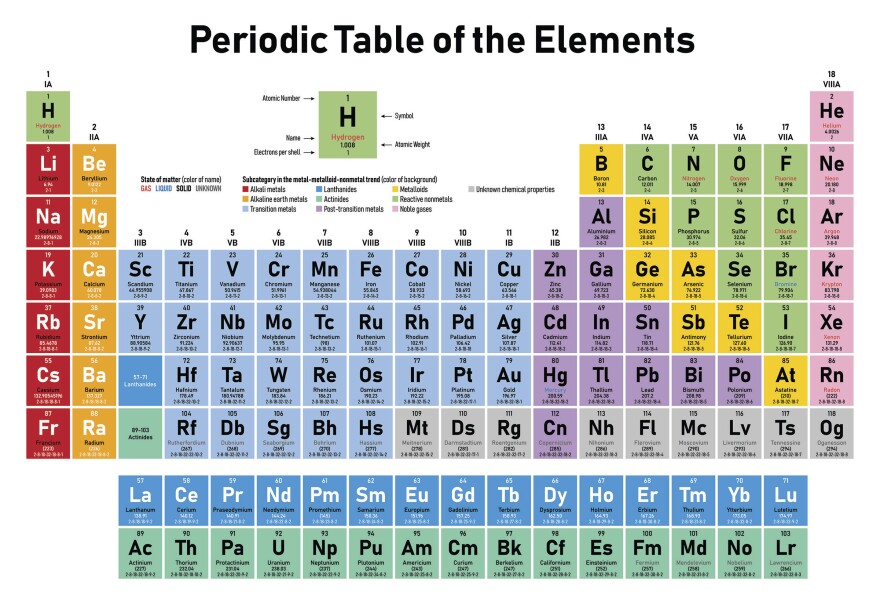
Using the periodic table (Valence Electrons – Periodic Table)
As the periodic table shows lots of important information about the elements, scientists use it to determine the number of valency by checking out the place of elements in the table depending on the facts “All elements in a single vertical column have the same number of valence electrons” and “The atom’s main group number is equal to the number of valence electrons for these atoms but ignore the Transition metals which are the elements located in the rectangle-shaped block made by Groups 3 to 12. “
So, just by checking the number of the group that contains the wanted element, we can know the number of valency for the element which is located in that specific column.
Sodium Valence Electrons
For example, we can find the valency for sodium (Na) which have an atomic number of 11. It is located at group 1. As a result, one atom of sodium has one valence electron.
Note: This method can’t be used with the transition metals which are located in groups number 3,4,5,6,7,8,9,10,11,12. The atomic structure of these elements is different, rigid and need a different way to calculate the number of valence electrons.
The following table shows the groups that we can find the number of valence electrons by using the periodic table method and the number of valence electrons for the elements that are located in each group:
| Periodic Table Group | Valence Electrons |
| Group 1 (I) – Alkali metals | 1 |
| Group 2 (II) – Alkaline earth metals | 2 |
| Group 13 (III) – Boron group | 3 |
| Group 14 (IV) – Carbon group | 4 |
| Group 15 (V) – Nitrogen group | 5 |
| Group 16 (VI) – Oxygen group | 6 |
| Group 17 (VII) – Halogens | 7 |
| Group 18 (VIII or 0)- Noble gasses
(Except Helium, which has 2 valence electrons) |
8 |
Let’s take some examples to apply the periodic table method to find the valence electrons
Nitrogen Valence Electrons
If we check nitrogen (N) in the periodic table, we will find it in group 15 (Vl) and by applying the fact “The atom’s main group number is equal to the number of valence electrons for these atoms”.
So, how many valence electrons does nitrogen have?
The number of nitrogen valence electrons is 5
Sulfur Valence Electrons
If we check sulfur (S) in the periodic table, we will find it in group 16 (V) and by applying the fact “The atom’s main group number is equal to the number of valence electrons for these atoms”.
So, the number of sulfur valence electrons is 6
Chlorine Valence Electrons
If we check chlorine (Cl) in the periodic table, we will find it in group 17 (VII) and by applying the fact “The atom’s main group number is equal to the number of valence electrons for these atoms”.
So, how many valence electrons does chlorine have?
The number of chlorine valence electrons is 7
Phosphorus Valence Electrons
If we check phosphorus (P) in the periodic table, we will find it in group 15 (Vl) and by applying the fact “The atom’s main group number is equal to the number of valence electrons for these atoms”.
So, how many valence electrons does phosphorus have?
The number of phosphorus valence electrons is 5
Fluorine Valence Electrons
If we check fluorine (F) in the periodic table, we will find it in group 17 (VII) and by applying the fact “The atom’s main group number is equal to the number of valency for these atoms”.
So, how many valence electrons does fluorine have?
The number of fluorine valence electrons is 7
Try PraxiLabs Virtual Chemistry Lab Experiments for FREE Now
More than 45 different and unique 3D science experiments are available
Electron Configuration
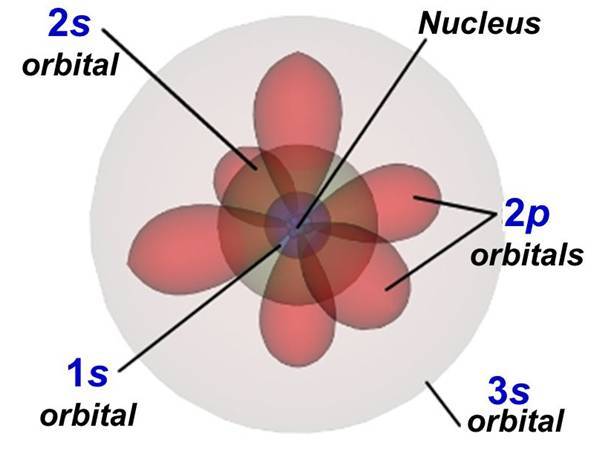
Another method to find valence electrons for elements is” the electron configuration”.
Electron configuration is the distribution of electrons of a molecule or atom in the orbitals (atomic or molecular).
For more understanding, let’s take an example for electron configuration:
Sodium (Na):
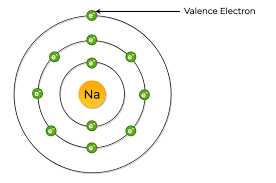
The electron configuration for sodium is 1s22s22p63s1, which means that sodium has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 6 electrons in the orbital “2p”.
- 1 electron in the orbital “3s”.
So the sodium total number of electrons is 11.
For more understanding, the following video explain the steps of electron configuration in details
Now, from the electron configuration you can find the number of electron valence by finding the number of electrons in the outermost shell which is equal to the number of valency.
Returning to our example “sodium Na”, the number of electrons in the outermost shell “3s” is one. So the number of sodium valence electrons is one.
Note: If the outer shell is full of electrons, in this case the element is called inert and will not react easily with other elements.
Let’s take some examples to apply the electron configuration method to find the valency
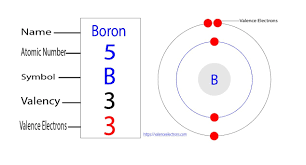
Boron Valence Electrons
The electron configuration for boron is 1s22s22p1, which means that boron has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 1 electron in the orbital “2p”.
So the boron total number of electrons is 5 and it has two shells: one with 2 electrons from “1s” orbital and other with 3 electrons from “2s and 2p” orbitals.
By applying the role “the number of electrons in the outermost shell is equal to the number of valency”, the number of electrons in the outermost shell “2s and 2p” is 3. So the number of boron valence electrons is 3.
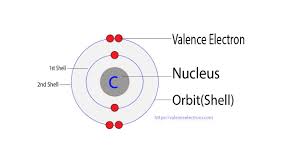
Carbon Valence Electrons
The electron configuration for carbon is 1s22s22p2, which means that carbon has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 2 electron in the orbital “2p”.
So the carbon total number of electrons is 6 and it has two shells: one with 2 electrons in the K-shell or the inner shell in the orbital 1s and other with 4 electrons in the L- shell or the outermost shell in the orbitals 2s and 2p.
How many valence electrons does carbon have?
By applying the rule “the number of electrons in the outermost shell is equal to the number of valence electrons, the number of electrons in the outermost shell “2s and 2p orbitals” is 4. So the number of carbon valence electrons is 4.
Potassium Valence Electrons
The electron configuration for potassium is 1s22s22p63s23p64s1 which means that sodium has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 6 electrons in the orbital “2p”.
- 2 electron in the orbital “3s”.
- 6 electrons in the orbital “3p”.
- 1 electron in the orbital “4s”.
So, the potassium total number of electrons is 19 and it has 4 shells. It has only one electron in the outermost shell in the orbital 4s.
How many valence electrons does potassium have?
By applying the role “the number of electrons in the outermost shell is equal to the number of valency”, the number of electrons in the outermost shell “4s orbital” is 1. So the number of potassium valence electrons is one.
Hydrogen Valence Electrons
The electron configuration for hydrogen is 1s1, which means that hydrogen has:
- 1 electrons in the orbital “1s”.
So the hydrogen total number of electrons is 1 and it has one shell which is the outermost shell in the orbital 1s.
By applying the role “the number of electrons in the outermost shell is equal to the number of valency”, the number of electrons in the outermost shell “1s orbital” is 1. So the number of hydrogen valence electrons is one.
Oxygen Valence Electrons
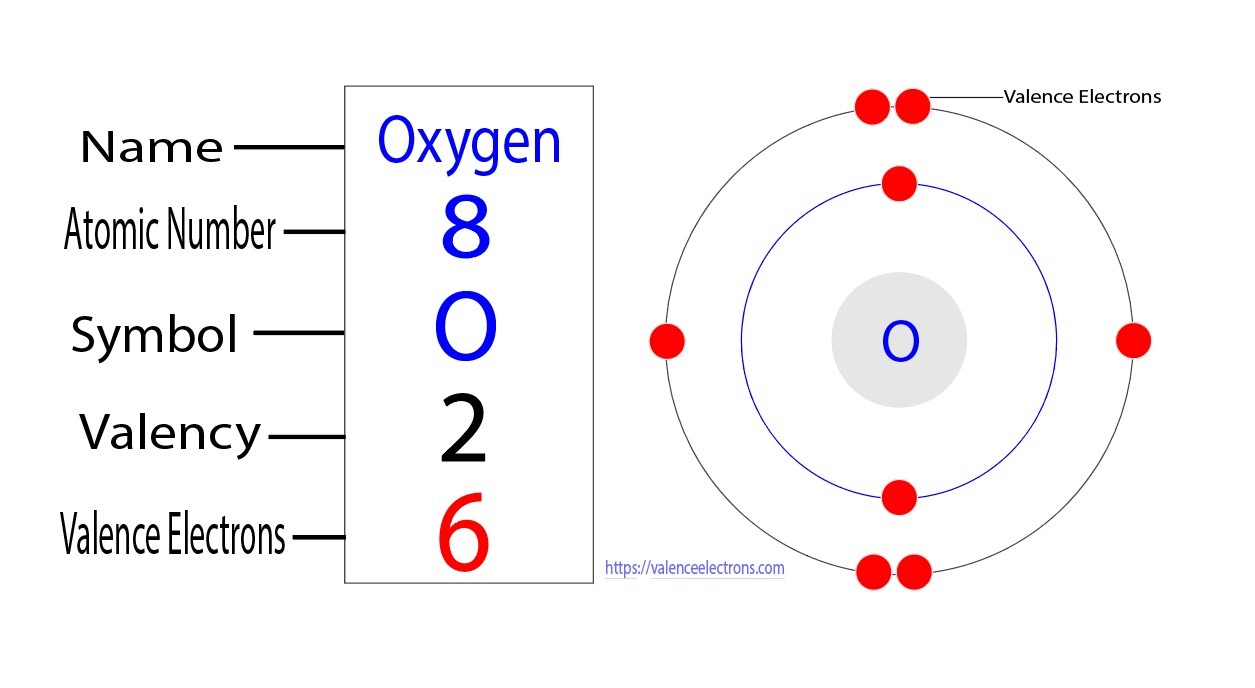
The electron configuration for oxygen is 1s22s22p4, which means that oxygen has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 4 electron in the orbital “2p”.
So the oxygen total number of electrons is 8 and it has two shells: one with 2 electrons in the K-shell or the inner shell in the orbital 1s and other with 6 electrons in the L- shell or the outermost shell in the orbitals 2s and 2p.
How many valence electrons does oxygen have?
By applying the role “the number of electrons in the outermost shell is equal to the number of valency”, the number of electrons in the outermost shell “2s and 2p orbitals” is 6. So the number of oxygen valence electrons is 6.
Calcium Valence Electrons
The electron configuration for calcium is 1s22s22p63s23p64s2 which means that calcium has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 6 electrons in the orbital “2p”.
- 2 electron in the orbital “3s”.
- 6 electrons in the orbital “3p”.
- 2 electron in the orbital “4s”.
So, the calcium total number of electrons is 20 and it has 4 shells. It has 2 electrons in the outermost shell (N-shell) in the orbital 4s.
By applying the role “the number of electrons in the outermost shell is equal to the number of valency”, the number of electrons in the outermost shell “4s orbital” is 2. So the number of calcium valence electrons is two.
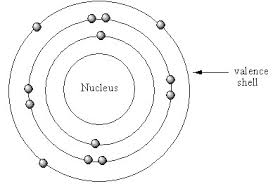
Aluminum Valence Electrons
The electron configuration for aluminum is 1s22s22p63s23p1 which means that calcium has:
- 2 electrons in the orbital “1s”.
- 2 electrons in the orbital “2s”.
- 6 electrons in the orbital “2p”.
- 2 electron in the orbital “3s”.
- 1 electrons in the orbital “3p”.
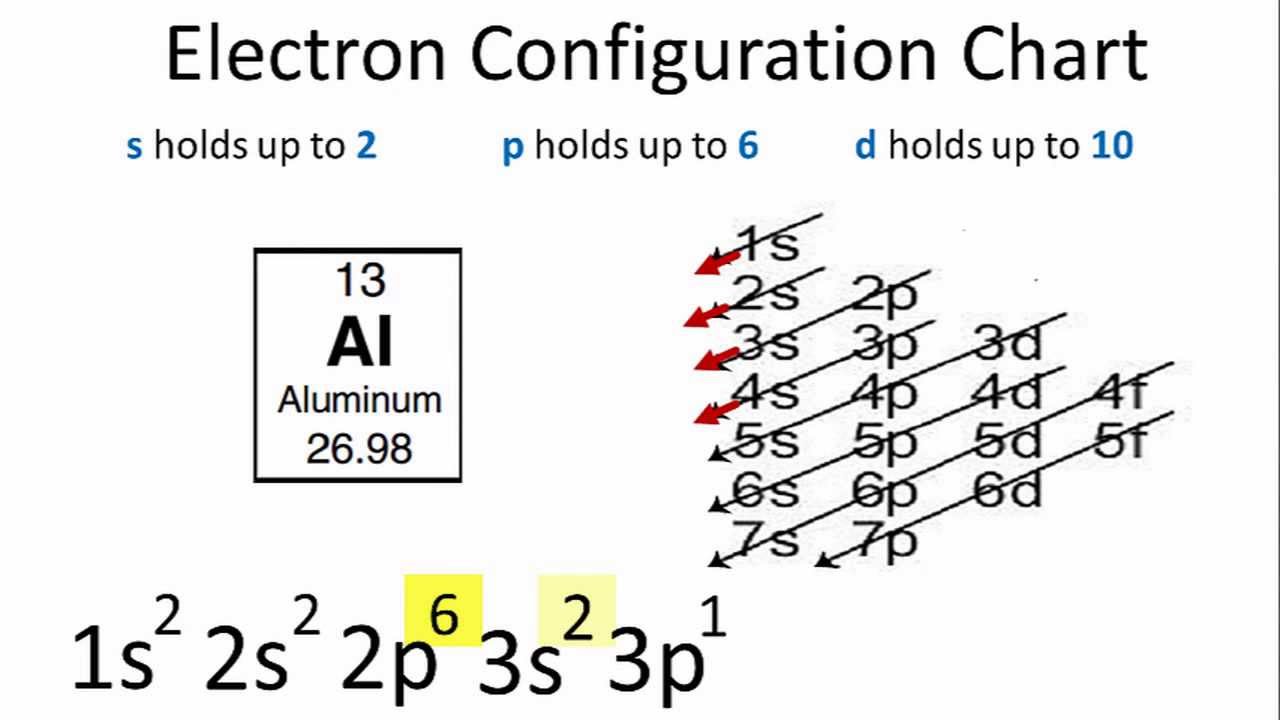
So, the aluminum total number of electrons is 13 and it has 3 shells. The first and the second shells (K and L shells) are filled with 10 electrons. It has 3 electrons in the outermost shell (M-shell) in the orbitals (3s and 3p).
By applying the role “the number of electrons in the outermost shell is equal to the number of valence electrons”, the number of electrons in the outermost shell “4s orbital” is 3. So the number of calcium valence electrons is 3.
Learn How to Calculate the Number of Valence Electrons for a Molecule
The number of valency for a molecule can be calculated by adding the valence electrons of all the atoms that form the molecule.
For more understanding, let’s take some examples:
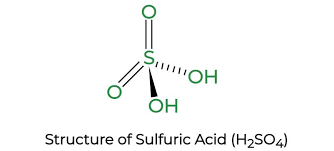
Sulfuric Acid H2SO4
One molecule of H2SO4 consists of:
- 2 Hydrogen atoms (1 valence electron for each hydrogen atom).
- 1 Sulfur atom (6 valence electrons for each sulfur atom).
- 4 Oxygen atoms (6 valence electrons for each oxygen atom).
So the total number of valency for one sulfuric acid molecule is (2*1+1*6+4*6) = 32
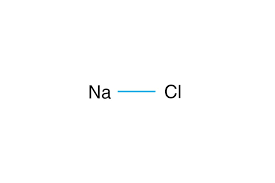
Sodium Chloride NaCl
One molecule of NaCl consists of:
- 1 Sodium atom (1 valence electron for each Sodium atom).
- 1 Chlorine atom (7 valence electrons for each Chlorine atom).
So the total number of valency for one Sodium Chloride molecule is (1+7) = 8
Unraveling the FAQs of Valence Electrons
How to calculate the valence electrons?
We can calculate the valence electrons by applying one of the following methods:
Periodic table
- Using the periodic table, we can determine the number of valence electrons by following these rules: All elements in a single vertical column have the same number of valence electrons.
- The atom’s main group number (group number excluding transition metals) indicates the number of valence electrons for these atoms. For example, elements in Group 1 have 1 valence electron, those in Group 2 have 2 valence electrons, and so on.“
We can find the valence electrons by applying the previous rules and observing the number of the group on the periodic table that contains the desired element.
Electron Configuration
Electron configuration refers to the distribution of electron in the orbitals (atomic or molecular).
After determining the electron configuration you can find the valence electrons by examining the number of electrons in the outermost shell (valence shell) which is equal to the number of valency.
What is the 2 8 8 18 rule in chemistry?
The 2 8 8 18 rule in chemistry refers to the electron filling rule or the electron configuration in the shells of an atom.
Electron Configuration: Electronic configuration is an arrangement of electrons in various shells, subshells and orbitals in an atom. It is written as the following pattern: 2, 8, 8, 18, 18, 32
Notation: It is written as nl x where:
- n indicates the principal quantum number
- I indicates the azimuthal quantum number or sub-shell
- x is the number of electrons.
Number of electrons : The number of electrons in n shell is given by the following equation:
2n2
e.g. in the first shell the number of electrons = 2.
Where to find the number of valence electrons on the periodic table?
We can find the number of valence electrons by observing the number of the group (vertical column) on the periodic table that contains the wanted element.
For more clarification, it is known that:
- Group 1 — 1 valence electron.
- Group 2 — 2 valence electrons.
- Group 13 — 3 valence electrons.
- Group 14 — 4 valence electrons.
- Group 15— 5 valence electrons.
- Group 16— 6 valence electrons.
- Group 17— 7 valence electrons.
- Group 18 — 8 valence electrons (except for helium, which has 2).
For example:
The valence electrons of carbon (C) is 4 because on the periodic table, carbon is located in group 14 and by applying the role “The atom’s main group number is equal to the number of valence electrons for these atoms”. So, the valence electrons of carbon is 4.
The valence electrons of lithium (Li) is 1 because on the periodic table, Lithium is located in group 1 and by applying the role “The atom’s main group number is equal to the number of valence electrons for these atoms”. So, the valence electrons of Lithium is 1.
If we check iodine (I) in the periodic table, we will find it in group 17 and by applying the fact “The atom’s main group number is equal to the number of valence electrons for these atoms”.
So, the number of Iodine valence electrons is 7.
Remember that the periodic table method for finding the valence electrons can’t be used with the transition metals which are located in groups no( 3,4,5,6,7,8,9,10,11,12) because the atomic structure of these elements is different and needs a special method to calculate their valence electrons.
How do you determine the number of valence electrons for beryllium?
We can determine the number of valence electrons for Beryllium by applying the periodic table method:-
If we check beryllium (Be) in the periodic table, we will find it in group 2 and by applying the role “The atom’s main group number is equal to the number of valence electrons for these atoms”.
So, the number of valence electrons for beryllium is 2.
On PraxiLabs you can find different virtual labs simulations in Chemistry accessible anytime, anywhere and 100% safe.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science