Last Updated on May 14, 2025 by Muhamed Elmesery
In life, nothing happens randomly or without reason. Even those events that you consider tiny are all fully dependent on the laws of nature. Everything happens for a reason, and according to firm laws, do not allow randomness to dominate the universe.
So, this article revolves around some applications that occur in our lives are thought to be random; however, they are based on one of the most important physical laws, which is Boyle’s law(real life applications of Boyle’s law) and why is Boyle’s law important .
At the end of the article, there will be a 3D simulation at the physics lab in PraxiLabs to prove Boyle’s law.

Table of Contents
Who is Boyle?
Boyle is a chemist and physicist and also an inventor. Although he suffered from a serious disease that affected his eyes permanently, he overcame his disability and hired some people to write his thoughts.
Boyle became the first to put the foundation stone in gases laws and to discover a law that reflected the behavior of gases. He discovered a breakthrough in physics which then became called Boyle’s law. He was also one of the founders of modern chemistry.
What is strange is that he did not officially attend any university. Boyle was so rich that he did all his research at his own expense. Despite his achievements in physics, his favorite subject was chemistry.
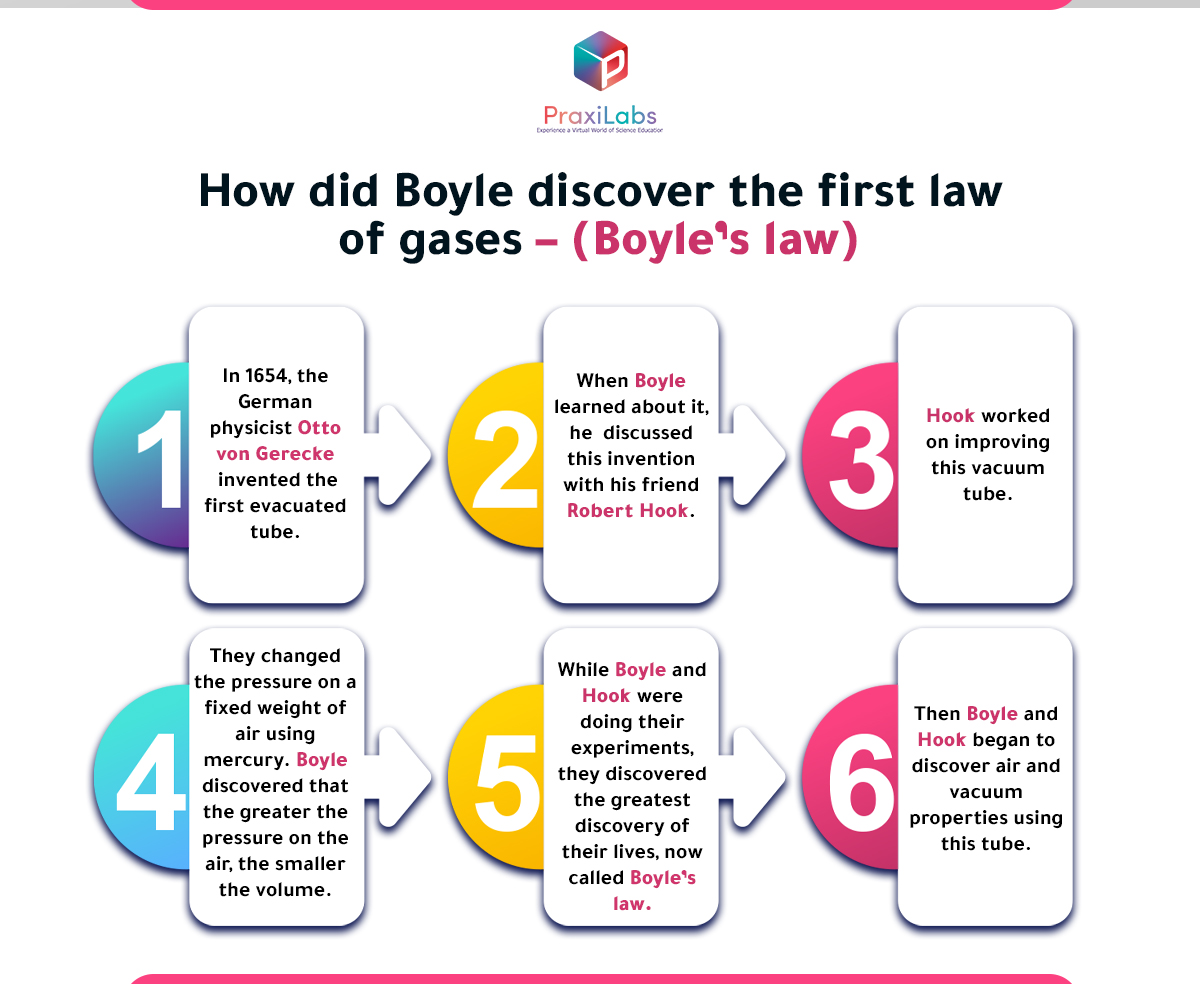
How did Boyle discover the first law of gases – (Boyle’s law)?
In 1654, the German physicist Otto von Gerecke invented the first evacuated tube. When Boyle learned about it, he discussed this invention with his friend Robert Hook.
Hook worked on improving this vacuum tube, then Boyle and Hook began to discover air and vacuum properties using this tube.
While Boyle and Hook were doing their experiments, they discovered the greatest discovery of their lives, now called Boyle’s law. They changed the pressure on a fixed weight of air using mercury. Boyle discovered that the greater the pressure on the air, the smaller the volume.
What is Boyle’s law and what is its significance? (significance of Boyle’s law)
When the pressure changes on a certain amount of gas, its size is inversely proportional to the pressure, provided that the temperature is constant.
The law is described by the mathematical equation PV = K. It has become a basic law in chemistry.
The Importance of Boyle’s law
The importance of Boyle’s law (boyle’s law of gases) lies in being the first law to describe the behavior of gases.
It explained that the gases spread in the medium, that is, the volume increases if the pressure is decreased and vice versa (the particles are displaced from each other and move easily) if the gas is compressed, causing the volume to shrink.
Create a FREE Virtual Labs Account Now!
Different Applications of Boyle’s Law in real life
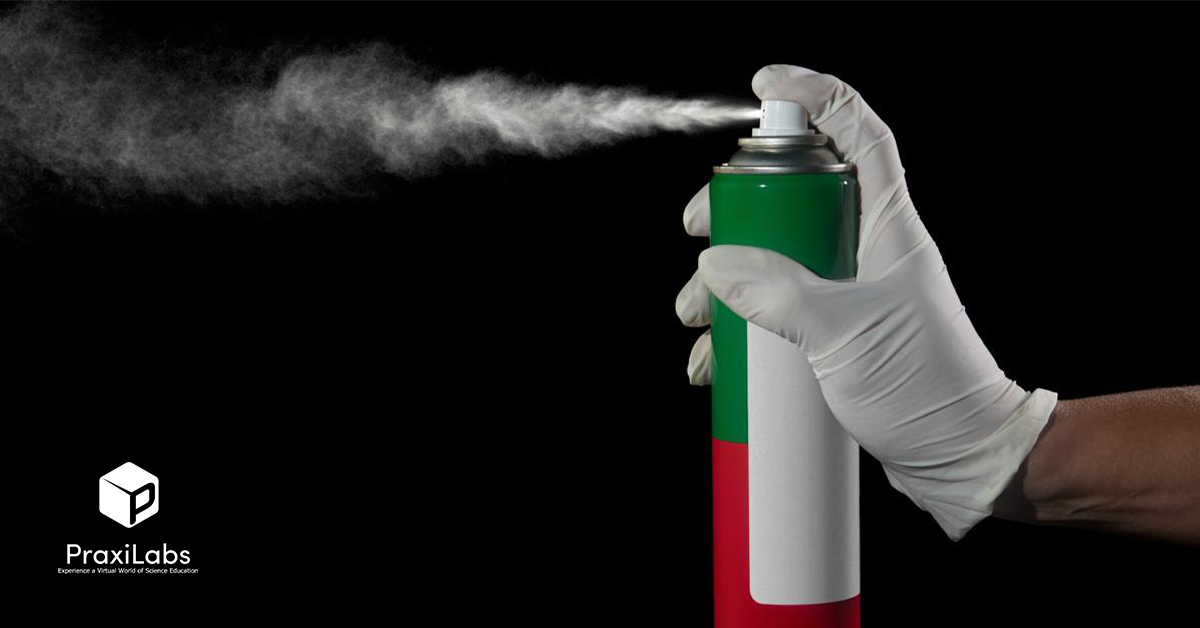
1- Spray paint Boyle’s law
You may have wondered how Boyle’s law is applied in aerosols?
Spray paint or aerosol spray is consider one of applications of Boyle’s law, as it is generally based on Boyle’s law, where the paint container contains two substances, one of them is the paint material itself, and the other is a compressed gas in a liquid state in the container. Although the liquefied gas boiling point is less than room temperature, it does not actually boil in the container and does not turn into gas because the container is best sealed.
As soon as you press the sprayer and the gas starts to get out of the container, the boiling state starts, the liquefied gas expands and turns into gas, and the gas presses the paint inside the container. The paint material is pushed up to get out of the sprayer nozzle with gas escaping from the container.
2- Soda bottle (Soda can Boyle’s law)
Soda bottles or cans are consider a practical application of Boyle’s law ,as all of us apply Boyle’s Law but unintentionally. Note that when you open the bottle of soda quickly, the gas rushes from everywhere in the form of foam, causing a mess. So What is the cause of this mess?
 This mess occurs because the soda is pumped into the soda bottle by passing the water on carbon dioxide. When you open the bottle, you are actually reducing the pressure on the gas, and the volume of the gas expands.
This mess occurs because the soda is pumped into the soda bottle by passing the water on carbon dioxide. When you open the bottle, you are actually reducing the pressure on the gas, and the volume of the gas expands.
If you remove the cap quickly, the gas pushes out of the bottle. Therefore, you should open the cap slowly and carefully until the gas comes out quietly.
We are facing another phenomenon in the cases of soda bottles, which is the effervescence of soda if the bottle exposed to shaking. So what happens in this case?
In this case, when the cap begins to open, the gas tries to escape from the bottle. But, because of being mixed with the liquid, the gas brings the fluid with it and they are pushed out together, turning into foam and causing a mess.

3- Diving into deep water
Every skillful diver knows that, after diving in deep water, divers have to return to the top very slowly. Our bodies are built and designed to live in natural pressure. Low and increased pressure cause many problems. Here is the scientific reason behind the slow rise.
As the diver moves down to the bottom of the water, the pressure increases. Increasing pressure leads to a decrease in volume, and the diver’s blood begins to absorb the nitrogen gas. The opposite happens when the diver starts to rise again, and the nitrogen gas molecules begin to expand and return to its volume.
If the diver makes a slow rise, the nitrogen gas molecules expand and return to normal without problems, but if it rises quickly, the diver’s blood turns into foam and the same mess that occurs in the soda bottles causes the diver to bend and feel strong pain.
In the worst case, this sudden drop in body pressure can instantly terminate the diver’s life.
Watch the following video, for a better explanation of changing of the volume as the pressure changes below the sea level.

An experiment to prove Boyle’s law at the physics lab in PraxiLabs.
After recognizing Boyle, his first law of gases and also applications of Boyle’s law , we thank you for your reading of the article to the end, and we invite you to try our virtual labs and do a 3D simulation to prove Boyle’s law via PraxiLabs.
Steps to access the experiment:
- Create a free account
- Click on My account
- Press 3D science simulations
- Click on Physics
- Select Boyle’s Law experiment
Finally, we wish you a pleasant experience with our virtual physics lab. Feel free to tell us how your experience was with PraxiLabs.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





