




2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
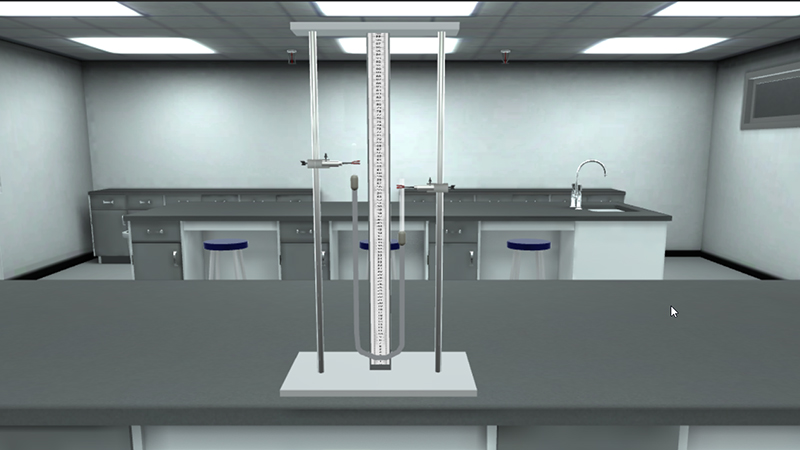
Verification of Boyle’s law by using gas law simulation lab.
J- shaped tube method
By the end of the Boyle's law simulation, student will be able to:
Fig 1. Fig 2.
In Boyle's law virtual lab, study the change in volume occupied by a gas when the pressure exerted on it changes at constant temperature..




