Last Updated on June 1, 2025 by Muhamed Elmesery
The XTT assay is an assay used for cellular metabolic activity measuring as an indicator of cell cytotoxicity, viability and proliferation. XTT assay stands for (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) which is a tetrazolium-based compound. XTT is a colorless or slightly yellow compound and becomes brightly orange when reduced. In this article we will explain XTT assay experiment virtual lab from PaxiLabs in detail, to understand the XTT assay protocol, principle, procedures, the results and the importance of the experiment. Before talking about the XTT assay experiment from PraxiLabs. Let’s talk an overview about cytotoxicity  Cytotoxicity is the toxicity caused by the chemotherapeutic agent’s action on living cells. Cytotoxicity can be caused by the drug itself, such as through the disruption of DNA synthesis, repair or transcription; or by its breakdown products or metabolites. Cytotoxicity tests are very important specifically in nanoparticles because they help in the determination of the proposed biomedical use. Cytotoxicity refers to the amount of damage a chemotherapeutic agent causes to cells. There are many methods involved for cytotoxicity and cell viability determination like:
Cytotoxicity is the toxicity caused by the chemotherapeutic agent’s action on living cells. Cytotoxicity can be caused by the drug itself, such as through the disruption of DNA synthesis, repair or transcription; or by its breakdown products or metabolites. Cytotoxicity tests are very important specifically in nanoparticles because they help in the determination of the proposed biomedical use. Cytotoxicity refers to the amount of damage a chemotherapeutic agent causes to cells. There are many methods involved for cytotoxicity and cell viability determination like: 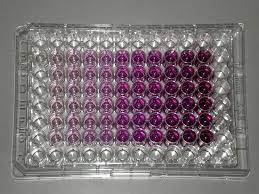
- Dyes that used to differentiate the various cells depending on the colors (based on the color uptake ratio of both living and dead cells). For example: Alamar Blue, trypan Blue and neutral red.
- MTT assay
- XTT assay
- Dehydrogenase-based assay
- WST assay
Cytotoxicity assays are common and used by the pharmaceutical industry for many vital purposes: Screening for cytotoxicity in compound libraries. In Nanotechnology, it can be used to look for cytotoxic nanomaterials and detecting if they are interested in developing a nanomedicine that targets rapidly dividing cancer cells. Cytotoxicity can also be monitored by measuring the reducing potential of the cells using a colorimetric reaction, or using ATP content as a marker of viability. Such ATP-based assays include bioluminescent assays in which ATP is the limiting reagent for the luciferase reaction. A label-free approach to follow the cytotoxic response of adherent animal cells in real-time provides the kinetics of the cytotoxic response rather than just a snapshot like many colorimetric endpoint assays.
Table of Contents
XTT Assay Principle
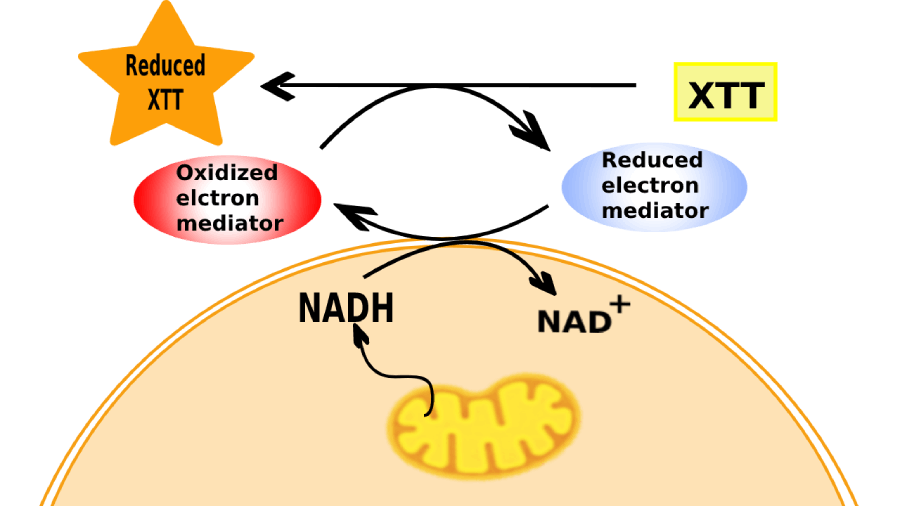 XTT assay principle depends on the fact that the mitochondria which are found in the cellular cytoplasm have many enzymes. Among these, a specific enzyme system called ‘succinate-tetrazolium reductase’ system that belongs to the mitochondrial respiratory chain and is only active in viable cells. XTT assay experiment aims to test the activity of the enzymatic system (the viability of cultured cells) and measures this activity in nanoparticles-treated cells in comparison with the control untreated-cells.
XTT assay principle depends on the fact that the mitochondria which are found in the cellular cytoplasm have many enzymes. Among these, a specific enzyme system called ‘succinate-tetrazolium reductase’ system that belongs to the mitochondrial respiratory chain and is only active in viable cells. XTT assay experiment aims to test the activity of the enzymatic system (the viability of cultured cells) and measures this activity in nanoparticles-treated cells in comparison with the control untreated-cells.
Uses of XTT Assay
To assay cell viability, cytotoxicity, or cell proliferation which is very common practice, we use tetrazolium salts, including XTT (2, 3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbox-anilide)
XTT proliferation assay
The XTT proliferation assay was first discovered in 1988 and it is now an effective method for measuring drug sensitivity and cell growth in tumor cell lines.
XTT biofilm assay
XTT biofilm assay or the tetrazolium salt assay is commonly used as a test to examine and determine the impact of biofilm therapies and estimate viable biofilm growth (XTT biofilm assay)
XTT Assay Protocol
XTT cell viability assay protocol aims to test the cultured cells viability after exposure to different nanoparticles with geometric concentration. In the presence of the succinate -tetrazolium reductase system in the active cells mitochondria, a Cleavage of the tetrazolium salt to formazan occurs. 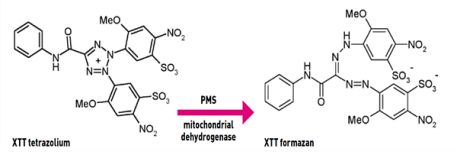 The yellow tetrazolium salt (XTT) is converted or cleaved to formazan a soluble orange dye and can be measured by absorbance at 490 (or 450) nm in a microplate reader.
The yellow tetrazolium salt (XTT) is converted or cleaved to formazan a soluble orange dye and can be measured by absorbance at 490 (or 450) nm in a microplate reader. 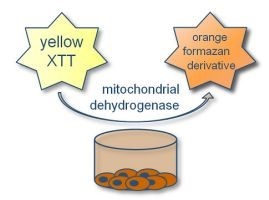
In Vitro Viability Assay Using Tetrazolium Salt XTT Simulation from PraxiLabs
 We provide 3D virtual lab simulation (In Vitro Colorimetric Analysis of Cell Viability by XTT Assay) for post-graduate students. By the end of the simulation they will be able to do and understand the following:
We provide 3D virtual lab simulation (In Vitro Colorimetric Analysis of Cell Viability by XTT Assay) for post-graduate students. By the end of the simulation they will be able to do and understand the following:
- Adding the XTT reagent to cells and reading the results by using the microplate reader after the process of cell incubation.
- Reading the results of XTT assay and calculating the percent of cell viability after exposure to different doses of tested chemical(s).
- Calculate the tested chemicals concentration and then prepare them in the cell culture medium.
- Remove the old medium and add the new medium that contains the tested chemicals in the appropriate wells.
- Count cells under the microscope and check the confluence.
- Try to dilute cells to a specific count that is suitable for seeding in the 96-well plate.
Method
In Vitro Colorimetric Analysis of Cell Viability by XTT Assay
Devices and Reagents Included in XTT Assay Experiment
Devices: Laminar airflow. Centrifuge. Microscope. CO2 incubator. Cell counting chamber (Neubauer Slide). Reagents & Consumables: T-25 tissue culture flasks. Variable automatic pipettes (1-10, 20-200, and 100-1000µl). 96-well plates. 8-multichannel pipette. Variable automatic pipettes (1-10, 20-200, and 100-1000µl). Pipette aid. Filter-tips (1-10, 20-200, and 100-1000µl). Sterile (or autoclaved) blue and yellow tips. DMEM Medium with phenol red, high glucose and L-glutamine. DMEM with high glucose and L- glutamine, without phenol red. Penicillin Amphotericin B solution. Streptomycin Trypsin 10x and Trypsin 1x. Fetal Bovine Serum (FBS). Sterile Phosphate Buffered Saline (PBS). Electron coupling agents, such as phenazine methosulfate (PMS). XTT sodium salt.
XTT Assay Procedures and Steps
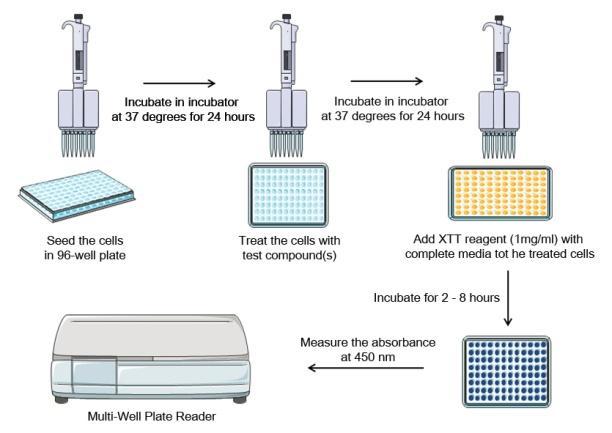 Performing XTT assay experiment takes about 3 days:- Day One:
Performing XTT assay experiment takes about 3 days:- Day One:
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Discard the used media of the T-25 tissue culture flask in the waste container.
- Wash cells by pipetting 5 ml of the balanced salt solution without calcium and magnesium to the side of the vessel opposite the attached cell layer to avoid disturbing the cell layer.
Gently, move the vessel back and forth several times. Note: The step of washing removes any traces of calcium, magnesium and serum that may inhibit the action of the trypsin dissociating reagent.
- Remove and discard the wash solution from the culture flask in the waste container.
Note: Repeat the wash step one more time.
- trypsinize one T-25 flask by adding 1 ml of trypsin/EDTA 1x solution to the T-25 flask.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Incubate the flask in a CO2 incubator for 5 minutes.
- After incubation, observe the trypsinized cells under the inverted light microscope to be sure that 90% of cells were already detached from the flask.
Note: In case of cells with less than 90% detached, the incubation time should be increased (few minutes), and check the dissociation every 30 seconds. Also, tap the vessel to speed up cell detachment.
- After cell detachment, add 5 ml of complete media to trypsinized cells to stop trypsinization.
- Collect the loosed and detached cells carefully by pipetting the medium up and down several times over the cell layer surface.
- Collect the trypsinized cells from the T-25 flask with a pipette and put them in a 15 ml falcon tube.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Put the falcon tube on the centrifuge at 1000 rpm for 5 minutes at 25 Celsius.
- Remove the supernatant media from the falcon tube by pipetting.
Note: Be careful to not disturb the pellet while removing the supernatant.
- Resuspend the pelleted cells in the falcon tube by adding 1 ml of complete media by up and down pipetting.
- In a new Eppendorf tube, take 10 µl of re-suspended cells and 40 µl trypan blue stain and mix by pipetting, then put 10 µl of mixed solution in Neubauer counting chamber.
Note: Remember to incubate the falcon tube in the CO2 incubator while counting the cells.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Count the cells in the peripheral 4 squares under an upright light microscope and calculate their mean count. Use in the following formula:
Cell count/ml = Mean of cell count (in the 4 large squares) x Dilution factor x 10E+04 (104) Example: if the counts of the 4 peripheral squares were 73, 75, 85, and 87, then their mean= (73+75+85+87) / 4 = 320/4 = 80. Total count of cells/ml = 80 * 5 * 104 = 400 * 104 (4 Million cells per milliliter).
- For seeding the cells in the 96-well plate, pipette 9.9 ml of complete media in a new tube and add to it 100 µl of cells (Contains 400,000 cells) and mix carefully.
- Pour the mixture into the pipette basin. Use an 8-channel pipette to aliquote 100 µl of re-suspended cells (4000 cells) into each well of the 96-well plate columns, except in the first column which will be considered as the Non-Cell Control (NCC).
Note: To avoid poor replicates among wells, ensure no bubbles are present inside the wells, check the accuracy of pipettes, and pipette cells volume accurately.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Incubate the whole plate in a CO2 incubator for 24-48 hours.
Day Two:
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- From 10 µg/µl stock solution of titanium oxide, silver, and iron oxide (magnetite) nanoparticles (NPs), prepare the following concentrations for each NP according to the following table. Take with a micropipette the desired volumes of stock solution and complete medium and mix them in a new tube for each concentration:
| Concentration of NPs | The volume of Stock Solution | The volume of Complete Medium | |
| 1. | 100 µg | 50 µl | 450 µl |
| 2. | 50 µg | 25 µl | 475 µl |
| 3. | 25 µg | 12.5 µl | 487.5 µl |
| 4. | 12.5 µg | 6.25 µl | 493.75 µl |
| 5. | 6.25 µg | 3.125 µl | 496.875 µl |
| 6. | Vehicle Control | 50 µl (Distilled Water) | 450 µl |
| 7. | NCC | —— | 500 µl |
- Take out the 96-well plate from the incubator. In the laminar airflow hood carefully aspirate the old media from plate wells by the 8-channel pipette.
- Aliquote 100 µl of each prepared nanoparticles concentrations into 4 wells only using a micropipette, according to the following scheme:
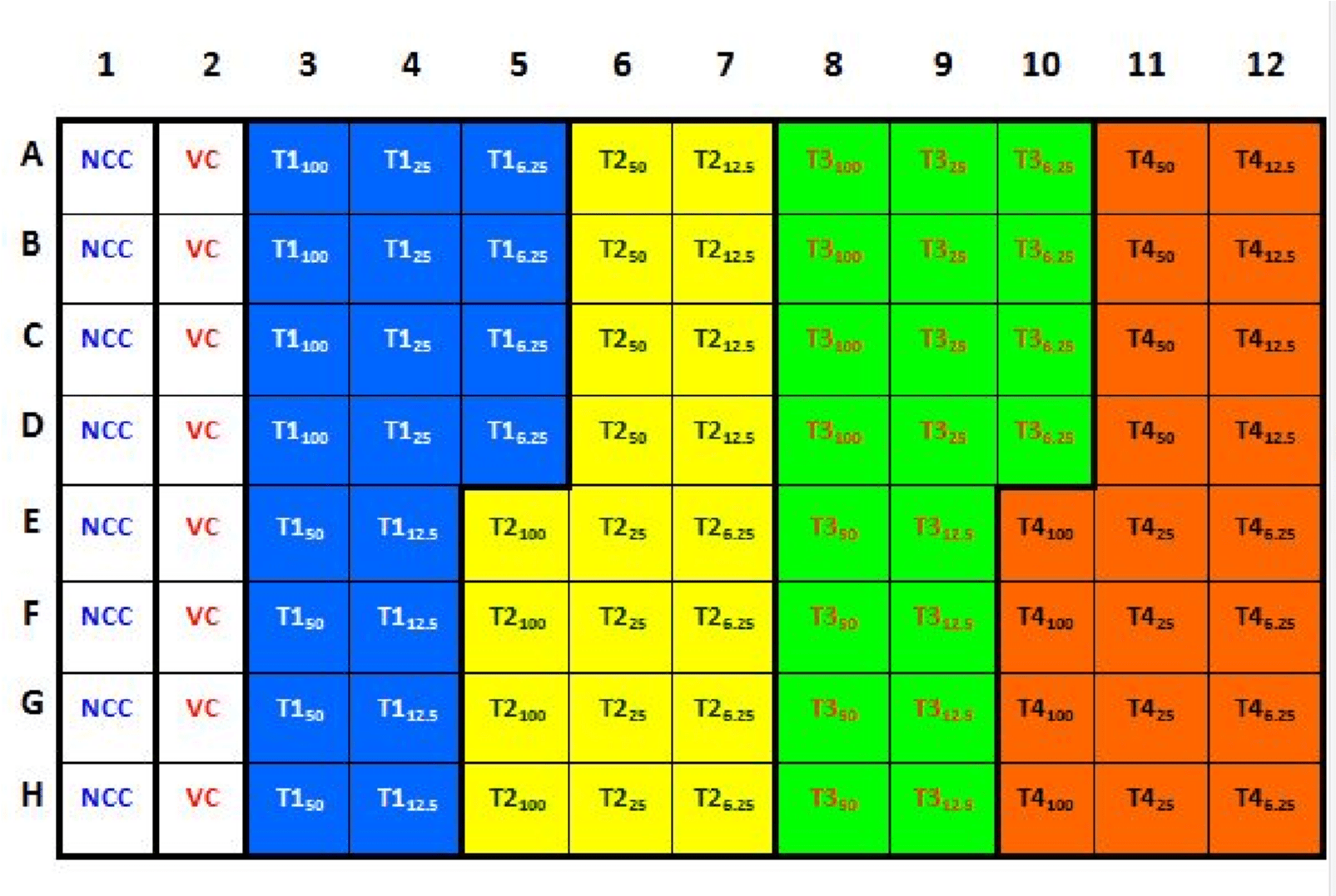 Where, (NCC = Non-Cell Control; Wells 1A to 1H), (VC = Vehicle Control; Wells 2A to 2H), (T1 = nano-zinc oxide treatment; Wells 3A to 5D), (T2 = nano-titanium oxide treatment; Wells 5E to 7H), (T3 = nano-silver treatment; Wells 8A to 10D), and (T4 = nano- iron oxide treatment 4; Wells 10E to 12H).
Where, (NCC = Non-Cell Control; Wells 1A to 1H), (VC = Vehicle Control; Wells 2A to 2H), (T1 = nano-zinc oxide treatment; Wells 3A to 5D), (T2 = nano-titanium oxide treatment; Wells 5E to 7H), (T3 = nano-silver treatment; Wells 8A to 10D), and (T4 = nano- iron oxide treatment 4; Wells 10E to 12H).
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Incubate the treated plate in the incubator for 24 -48 hours.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
Day Three:
- Remove cultures from the incubator into a laminar flow hood.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Prepare XTT Working Solution immediately before use by adding and dissolving 5 mg XTT salt in 5 ml of complete medium (1 mg/ml) inside a falcon tube.
- Prepare the XTT working solution by adding 10 µl of phenazine methosulfate (electron coupling agent) to the XTT salt solution prepared in the previous step.
- Add 50 uL of XTT working solution to each well of the 96-well plate using the multichannel pipette.
- Before work, clean the work surface of the laminar airflow with 70% ethanol.
- Cover the plate and place it inside the microplate reader. Read absorbance at 450 nm using a multi-well plate reader, with a reference wavelength of 690 nm (to correct for fingerprints, smudges, etc. on the plate lid).
Save your time now and try the simulation!
Results
The student takes out the microplate from the multi-well plate reader. The microplate should look like the following: 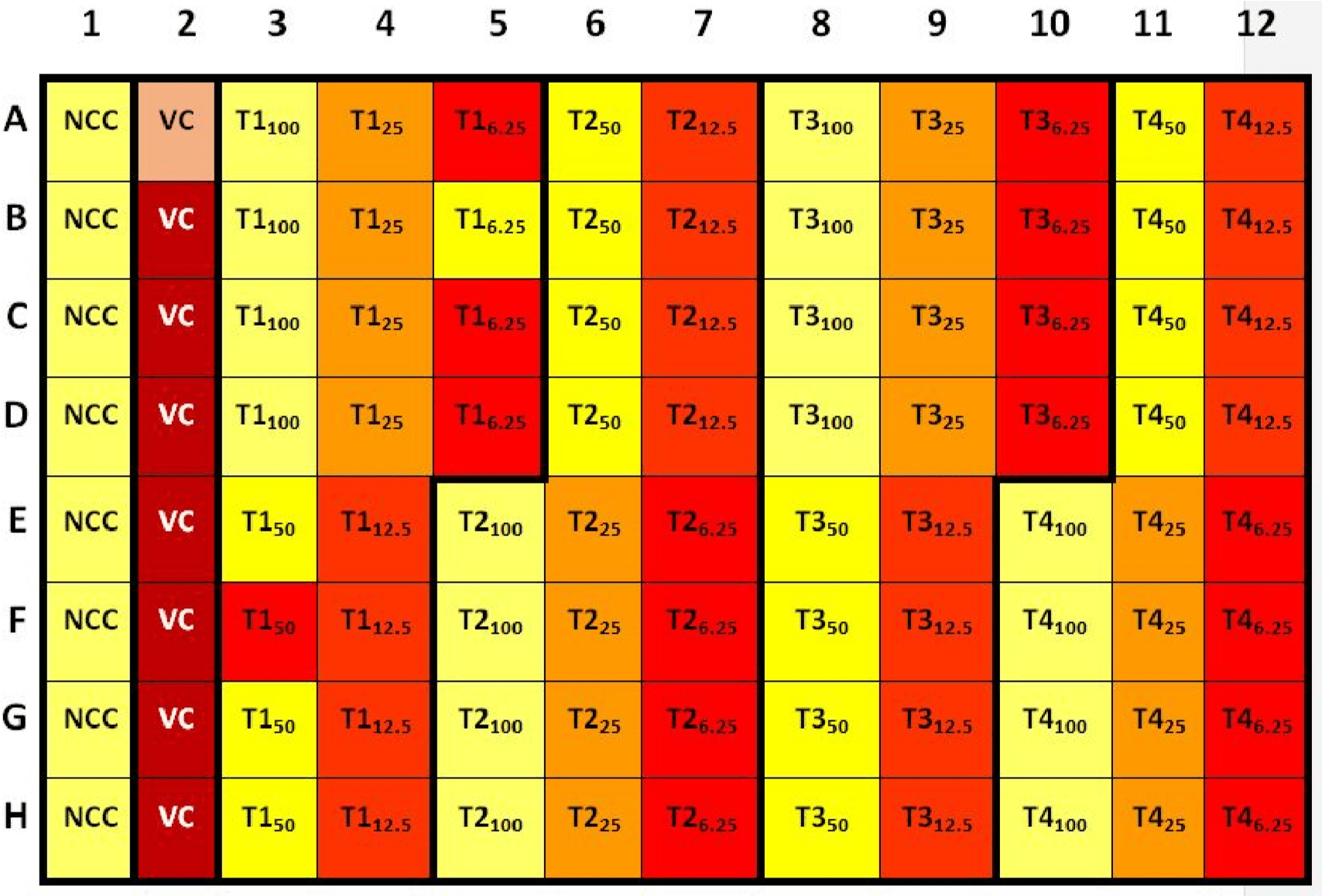
Calculation
From the absorbance readings of the nano-zinc oxide treatment, calculate the viability according to the following equation: Averaged absorbance of each set of treated samples Viability %= —————————————————————————- x 100 Averaged absorbance of Vehicle control (VC) samples Remember that:
- The lower viability percentages point to how toxic the concentration of tested nanoparticles on the tested cells.
- The dose of 50% viability percent is considered as the LC50 of tested nanoparticles on certain cell lines.
- The much higher viability percentages point to how much safer the nanoparticles are in vitro.
- If the blanks absorbance is high, this may mean that the culture medium contains a reducing agent and in this case an alternative medium should be used.
Create your free account now and try our virtual lab simulations that come with exciting features to make Learning Accessible for All Learners and to drive more value!
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





