4_2_2023_9_02_58_AM.jpg)
4_2_2023_9_02_58_AM.jpg)
4_2_2023_9_02_58_AM.jpg)
4_2_2023_9_02_58_AM.jpg)


2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Determination of acetyl salicylic acid concentration using aspirin titration experiment.
Determination of concentration of the active ingredient (acetyl salicylic acid) abundant in aspirin through back acid base titration.
By the end of aspirin Titration Online Simulation, students will:
C 9 H 8 O 4 + Na OH → Na C 9 H 7 O 4 + H 2 O
acetylsalicylic acid sodium hydroxide sodium acetylsalicylate water
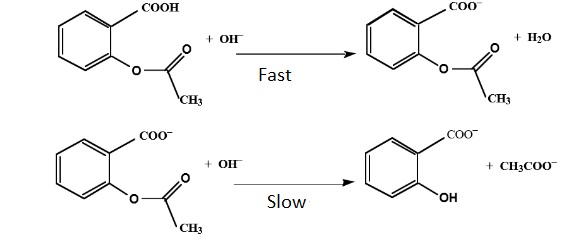
Figure 1. Reaction between acetyl salicylic acid and NaOH
In summary, the titration of aspirin allows accurate determination of the active ingredient in commercial tablets despite the presence of inactive components.
At aspirin titration experiment:




