Claisen Schmidt Reaction (Mixed Aldol Condensation)
Chemistry | Organic Chemistry
4_2_2023_6_43_58_PM.jpg)
4_2_2023_6_43_58_PM.jpg)
4_2_2023_6_43_58_PM.jpg)
4_2_2023_6_43_58_PM.jpg)


2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Preparation of dibenzalacetone through Claisen Schmidt condensation reaction.
⦁ DBA and its analogs can be synthesized from a classic Claisen Schmidt reaction (cross-aldol) condensation between acetone and benzaldehyde derivatives, with the reaction mechanism shown in Scheme 2. ⦁ Under the basic condition, hydroxide ion deprotonates acetone 2 at its a-position to form an enolate. ⦁ This nucleophilic enolate reacts with 4-fluorobenzaldehyde 1 to form a b-hydroxy ketone, which readily undergoes dehydration to produce intermediate 3. ⦁ Since acetone can be deprotonated at both its a-position to form reactive enolate species, one acetone molecule can react consecutively with two benzaldehyde molecules to form the symmetrical DBA product.
Become proficient at running organic chemical reactions:
The Claisen-Schmidt reaction (crossed-aldol reaction) is a condensation reaction of aldehydes and carbonyl compounds leading to β-hydroxycarbonyl compounds and it has played an important role in synthetic organic chemistry.
Subsequent dehydration of the β-hydroxycarbonyl compounds affords α-alkylidene or α-arylidene compounds.
Therefore, the Claisen-Schmidt condensation reaction is an organic reaction in which a ketone or an aldehyde holding an α-hydrogen reacts with an aromatic carbonyl compound which does not have any α-hydrogens to explain condensation reaction.
Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon-carbon bonds.
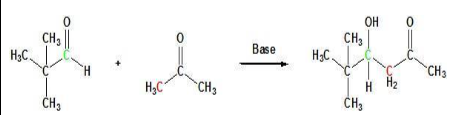
The general equation describes the Claisen Schmidt reaction (crossed aldol condensation)
This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. G.
Schmidt, who independently published on this topic in 1880 and 1881.
An example is the synthesis of dibenzylideneacetone ((1E, 4E)-1,5-diphenylpenta-1,4-dien-3-one).
The mechanism of the Claisen condensation reaction proceeds with the removal of an alpha proton through the action of a strong base to result in the formation of an enolate ion.
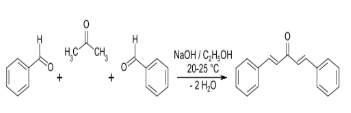
Dibenzalacetone synthesis
In every case, the product results from the addition of one molecule of an aldehyde (or ketone) to a second aldehyde (or ketone) in such a way that the α-carbon (in the form of an enolate ion) of the first becomes attached to the carbonyl carbon of the second. This reaction is depicted below.
Although a β-hydroxyaldehyde (or a β-hydroxyketone) is produced in an aldol condensation, the ultimate product of these reactions (as shown above) is usually the α, β-unsaturated aldehyde (or ketone) and a separate molecule of water.
Upon heating, the β-hydroxy aldehyde (or ketone) product of an aldol condensation easily undergoes dehydration to yield an α, β-unsaturated aldehyde (or ketone).
Conjugation of the newly formed double bond with the carbonyl group (or of the benzene ring, as shown below) stabilizes the unsaturated product and provides the thermodynamic driving force for the dehydration process.
Claisen-Schmidt condensation or (a mixed, or crossed aldol condensation) involving an aromatic aldehyde.
The Claisen-Schmidt condensation always involves dehydration of the mixed aldol condensation product to yield a chemical in which the double bond (produced during dehydration) is conjugated to both the aromatic ring and the carbonyl group.
Because this aromatic aldehyde lacks α-hydrogens, only one product can be formed, rather than a mixture of four different compounds, as long as the concentration of the second aldehyde is carefully controlled.
The equilibrium is shifted toward the product because the compound precipitates from the reaction mixture as it is formed.
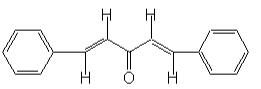
1, 5-diphenyl-1, 4-pentadien-3-one
First, aldehydes are more reactive acceptor electrophiles than ketones, and formaldehyde is more reactive than other aldehydes.
Second, aldehydes lacking alpha-hydrogens can only function as acceptor reactants, and this reduces the number of possible products by half.
The Claisen condensation reaction requires at least one of the reagents used to be enolizable. Therefore, they must have an α-proton which can be deprotonated for the formation of the enolate ion.
Another key requirement for this organic reaction is that the base used must not undergo nucleophilic addition or nucleophilic substitution and hinder the reaction.
The first part of this reaction is an aldol reaction, and the second part is dehydration (an elimination reaction).
Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present.
The aldol addition product can be dehydrated via two mechanisms; a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride in an enolate mechanism, or in an acid‐catalyzed enol mechanism.
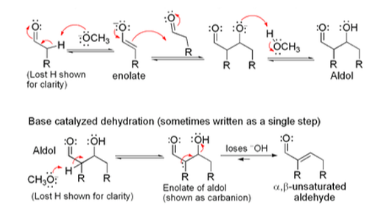
Step 1: The hydroxide ion deprotonates the enolizable aldehyde reversibly.
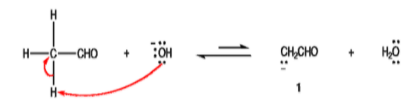
Step 2: Enolate ion aldehyde 1 preferentially adds to the non‐enolizable, which has the sterically less hindered and, therefore, more accessible carbonyl carbon.
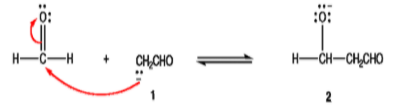
Step 3: Alkoxide ion 2 is protonated by water.

Step 4: Aldol 3 is an enolizable aldehyde. A small amount of it is converted to the corresponding enolate ion (4) by the hydroxide ion in the dibenzylideneacetone mechanism.

Step 5: Enolate ion 4 loses a hydroxide ion.
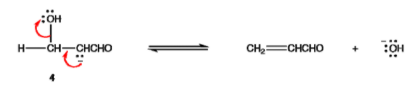
Steps 1 through 3 are a crossed aldol reaction, and steps 4 and 5 are a 1, 2 elimination via E1cB mechanism, following the claisen condensation mechanism steps.
Thus, crossed aldol condensation is crossed aldol reaction followed by 1, 2 elimination.




