Titration of Citric Acid in Juice by Acid/Base Titration
Chemistry | Analytical Chemistry






2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Determination of concentration of citric acid using titration curve in apple juice sample
Determination of concentration of citric acid in an apple juice sample using acid base titration with phenolphthalein indicator. Citric acid determination by titration: measuring the concentration of citric acid in an apple juice sample using acid-base titration with phenolphthalein indicator.
Identify the difference between acids and bases.
Acids can be classified, according to the number of ionizable hydrogen atoms they possess, into:
HCl + NaOH → NaCl + H2O
HNO3 + NaOH → NaNO3 + H2O
They can be sub-classified into:
H2SO4 + 2 NaOH → Na2SO4 + 2 H2O
H3PO4 + 3 NaOH → Na3PO4 + 3 H2O
C3H5O(COOH)3 + 3 NaOH → Na3C3H5O(COO)3
Note: If the excess volume of NaOH was withdrawn from the burette beyond the endpoint, the color of the sample in the flask will turn into an intense pink color due to the high basicity as shown in Figure 1c.
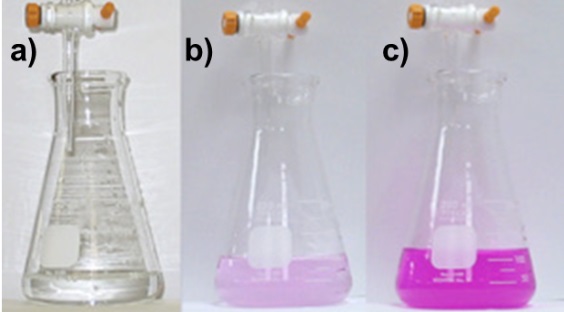
Figure1. Change of phenolphthalein color according to the medium
Determination of the concentration of citric acid in apple juice sample through titration of citric acid of the tested sample using phenolphthalein indicator, where titration of citric acid in juice provides accurate quantitative analysis.