Electrophilic Substitution Reaction - Azo Coupling Reaction
Chemistry | Organic Chemistry






2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Diazotization of phenolic and aromatic amines to study the electrophilic aromatic substitution mechanism and understand how electrophilic aromatic substitution effects influence reaction outcomes.
Azo coupling reactions are used for the diazotization of phenols and aromatic amines to produce azo dyes, and some sulfa drugs.
Become proficient at running organic chemical reactions through electrophilic aromatic substitution experiment.
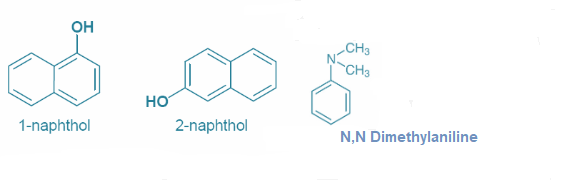
Figure 1. Diazonium ion formation.
In the electrophilic substitution reaction experiment:

1-Naphthol 2-Naphthol N,N-dimethylaniline




