2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
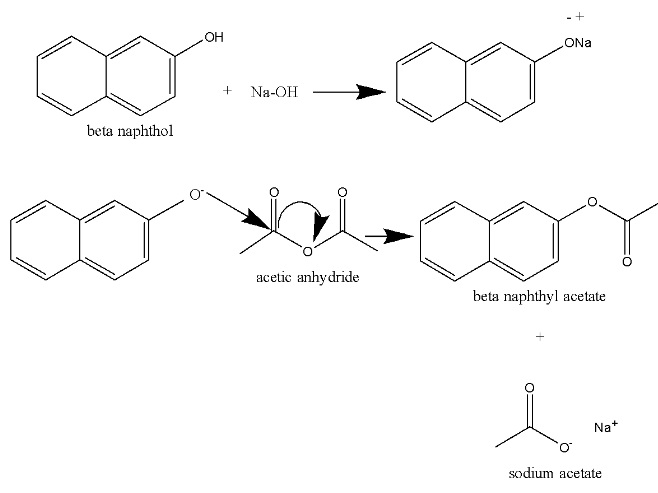
Synthesis of beta naphthyl acetate.
⦁ β-naphthyl acetate is prepared by chemical synthesis from β-naphthol, through acetylation with acetic anhydride in alkaline media. ⦁ The hydroxyl group on the β-naphthol forms an ester with the carboxyl group on the acetic anhydride.
Become proficient at running organic chemical reactions involving the naphthyl group.
Acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl functional group into a chemical compound. Such compounds are termed acetate esters or acetates.
Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions
In addition, acetylation is a chemical reaction in which an acetyl group is added to a compound in place of a hydrogen atom.
An acetyl group is a chemical functional group composed of a carbonyl group (a carbon double-bonded to an oxygen) and a methyl group (a carbon bound to three hydrogens).
Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound and is replaced by a hydrogen atom.
Acetyl group protonation
Nucleophilic attack
Leaving group departure.
The mechanism of acetylation begins with the protonation of the acetyl group. Next, the nucleophile (attacks the carbon center of the protonated acetyl group. Finally, the departure of the leaving group begins.
The new acetic anhydride-bound compound is acidic, and readily donates the proton back to the conjugate base in the solution.
The acid protonates the leaving group (the central oxygen of the acetic anhydride, now a carboxylic acid leaving group).
A lone pair of electrons on the acetyl alcohol replaces those bonding the positively-charged carboxylic acid, and the electron pair bonding the carboxylic acid to the acetyl group now resides solely on the carboxylic alcohol.
As the electrons bonding the carboxylic acid to the acetyl group reside solely on the carboxylic alcohol, the carboxylic acid is free to separate in the solution.
In the final step, a conjugate base removes a proton from the acetyl group to form the neutral acetylated product.
Generally, acetic anhydride is preferred over acetic acid in the acetylation reaction for beta naphthyl acetate. Acetic anhydride is more reactive and gives better yields of product.
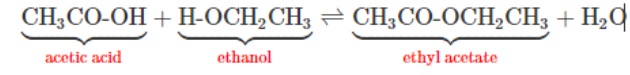
To get a good yield of ester you must use a large excess of acetic acid and/or remove the water as it is formed. While in the case of acetic anhydride:
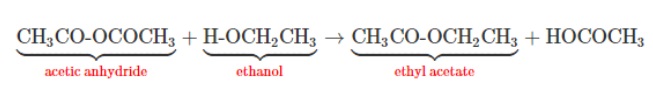
β Naphthyl acetate (2-Naphthyl acetate or 2-Acetoxynaphthalene) is a white to faint pink solid with the molecular formula of C12H10O2. The chemical structure of β Naphthyl acetate is illustrated below:
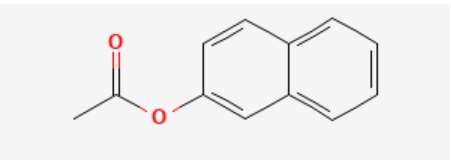
Figure 1. The chemical structure of β-naphthyl acetate
β-naphthyl acetate molecular weight is 186.21 g/mole and has IUPAC nomenclature of (naphthalen-2-yl acetate).
β-naphthyl acetate is used as a substrate in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to examine the composition of lipases in larvae and adult specimens, in the preparation of zymograms to identify proteins with lipase activity and to measure non-specific esterase activity and β-esterase enzyme activity.
β-naphthyl acetate is prepared through the acetylation of β-naphthol by acetic anhydride in alkaline media.
β-naphthol is a slightly acidic compound insoluble in water with a pKa value of 9.5.
The addition of NaOH converts β-naphthol into sodium salt of naphthols, called naphtholates, which are soluble in water.
The beta naphthyl acetate preparation through acetylation of β-naphthol by acetic anhydride is an example of a nucleophilic substitution exothermic reaction.