Preparation of Paracetamol | Synthesis of Paracetamol
Chemistry | Organic Chemistry






2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Synthesis of paracetamol. In this section, the preparation of paracetamol is introduced within the broader context of organic synthesis.
Paracetamol can be mainly prepared through addition-elimination reaction; where an aminol group of p-aminophenol reacts with the carboxylic group of acetic anhydride to form paracetamol and acetic acid. This reaction is catalyzed by the presence of concentrated acids (as sulfuric acid) to force the reaction forward, preventing it from going backward. This reflects the fundamental approach used in the laboratory preparation of paracetamol.
Become proficient at running paracetamol chemical reaction during the preparation of paracetamol process.
The general mechanism of the reaction between amino groups and the carboxylic group could be summarized as Follow:
Paracetamol has the molecular formula of C8H9NO2 as illustrated in the following structure:
The general equation of paracetamol synthesis is displayed in the following scheme:
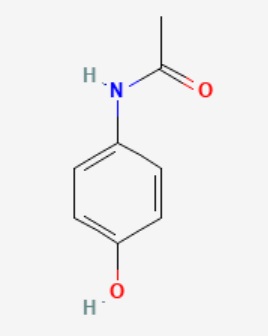
Figure 1. Chemical Structure of Paracetamol
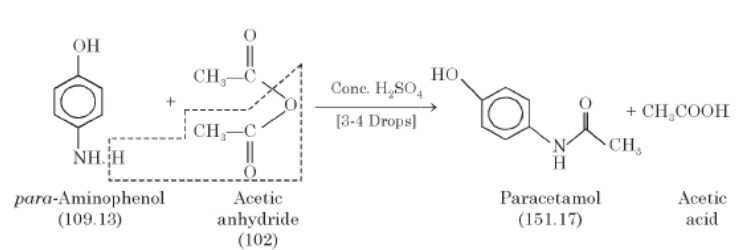
Figure 2. Schematic Diagram for the Paracetamol Synthesis Reaction
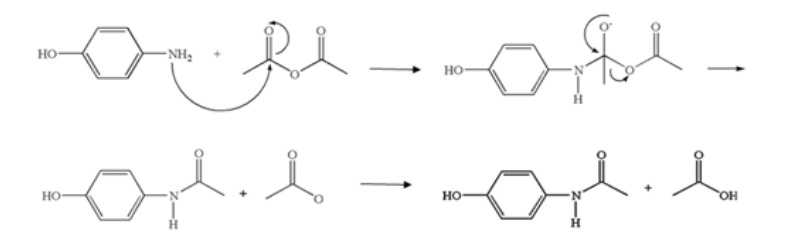
Figure 3. Mechanism of Paracetamol Synthesis Reaction




