





2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Saponification reaction and soap formation.
⦁ The aim of this saponification reaction experiment is to understand saponification reaction and how to prepare soap. Soap will be prepared through the alkaline hydrolysis of coconut oil. ⦁ The prepared soap will be purified by concentrated sodium chloride solution in the salting-out process.
Become proficient at running organic chemical reactions.
Soaps are an integral part to maintain good health and hygiene of the individuals.
Soaps are essential to cleanse dirt and oil off the objects including skin surface.
Soaps are widely used in bathing, cleaning, washing and in other household tasks.
Soaps are potassium or sodium salts of long-chain fatty acids.
The process of making soap is called saponification.
Saponification is the hydrolysis of a fatty acid ester with NaOH or KOH to give alcohol and sodium or potassium salt of the acid.
Depending on the nature of the alkali used in their production, soaps have distinct properties.
Sodium hydroxide (NaOH) produces "hard soap"; hard soaps can also be used in water containing Mg, Cl, and Ca salts. By contrast, potassium soaps (derived using KOH) are soft soap.
In addition, the fatty acid source also affects the soap's melting point.
During saponification reaction, ester reacts with an inorganic base (Lye) to produce alcohol and soap.
Generally, it occurs when triglycerides are reacted with potassium or sodium hydroxide to produce glycerol and fatty acid salt, called ‘soap’.
By using different types of alkali in the process the type of reaction product can be altered between hard and soft.
Triglycerides are generally animal fats and vegetable oils. When they are reacted with sodium hydroxide, a hard form of soap is created.
This is where potassium comes in and creates a softer version of the soap.
Ester + Base ————–> Alcohol + Soap
Step 1: Orthoester formation (Nucleophilic attack by the hydroxide)
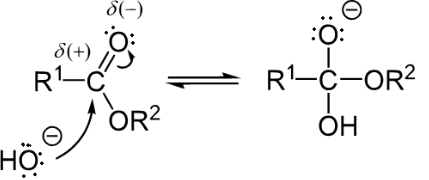
Step 2: Expulsion of carboxylic acid and alkoxide (Leaving group removal)
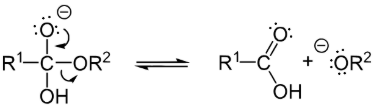
Step 3: Creation of alcohol (Deprotonation)
The alkoxide ion is a strong base so that the proton is transferred from the carboxylic acid to the alkoxide ion creating an alcohol.
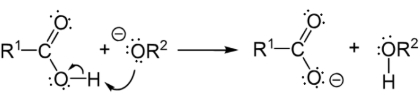
In a saponification reaction, a base (for example sodium hydroxide) reacts with any fat to form glycerol and soap molecules.
One of the saponification reaction is taking triglyceride as an ester and sodium hydroxide as the base.
In this saponification reaction, triglyceride reacts with sodium hydroxide (a strong base) and glycerol is produced (an acid) along with soap (sodium palmitate) as follows:
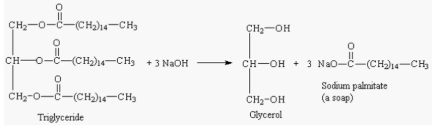
Similarly, potassium soap can be formed if a strong potassium base (like KOH) is reacted with an ester.
This reaction is as follows:
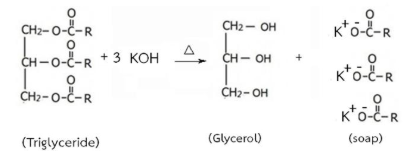
Saponification process can be either one-step saponification or two-step saponification process to convert triglycerides to soaps.
The examples mentioned above are a one-step saponification process in which triglycerides, when treated with a strong base, splits from the ester bond to release glycerol and soaps (i.e. fatty acid salts).
On the other hand, in the two-step saponification process, the steam hydrolysis of the triglyceride yields glycerol and carboxylic acid (rather than its salt). In the second step, alkali neutralizes fatty acid to produce soap.
Understanding the saponification test procedure is essential for both saponification lab work and industrial soap production applications.
This saponification experiment provides hands-on experience with one of the most important organic chemistry reactions.




