




2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
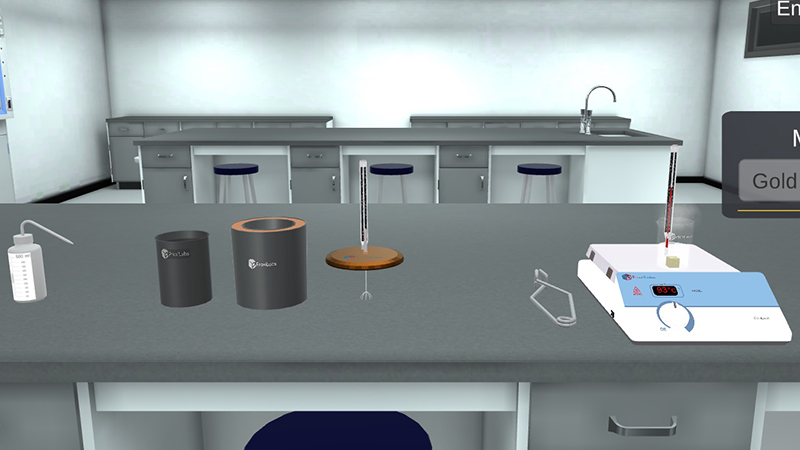
Determination of specific heat capacity of different materials through a simulation understanding specific heat.
Mixtures method in guided specific heat of solid lab.
By the end of the specific heat experiment, student will:
Heat lost by hot body = heat gained by the colder body
During the specific heat of solids experiment:




