Strong Acid Base Titration Virtual Lab (HCI/NaOH)
Chemistry | Analytical Chemistry





2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
Determination of HCl molar concentration in acid base titration virtual lab.
Determination of hydrochloric acid concentration using titration with sodium hydroxide in the acid base titration virtual lab.
Gain the knowledge of how acids and bases will react if their formulas are known in the acid base titration virtual lab.
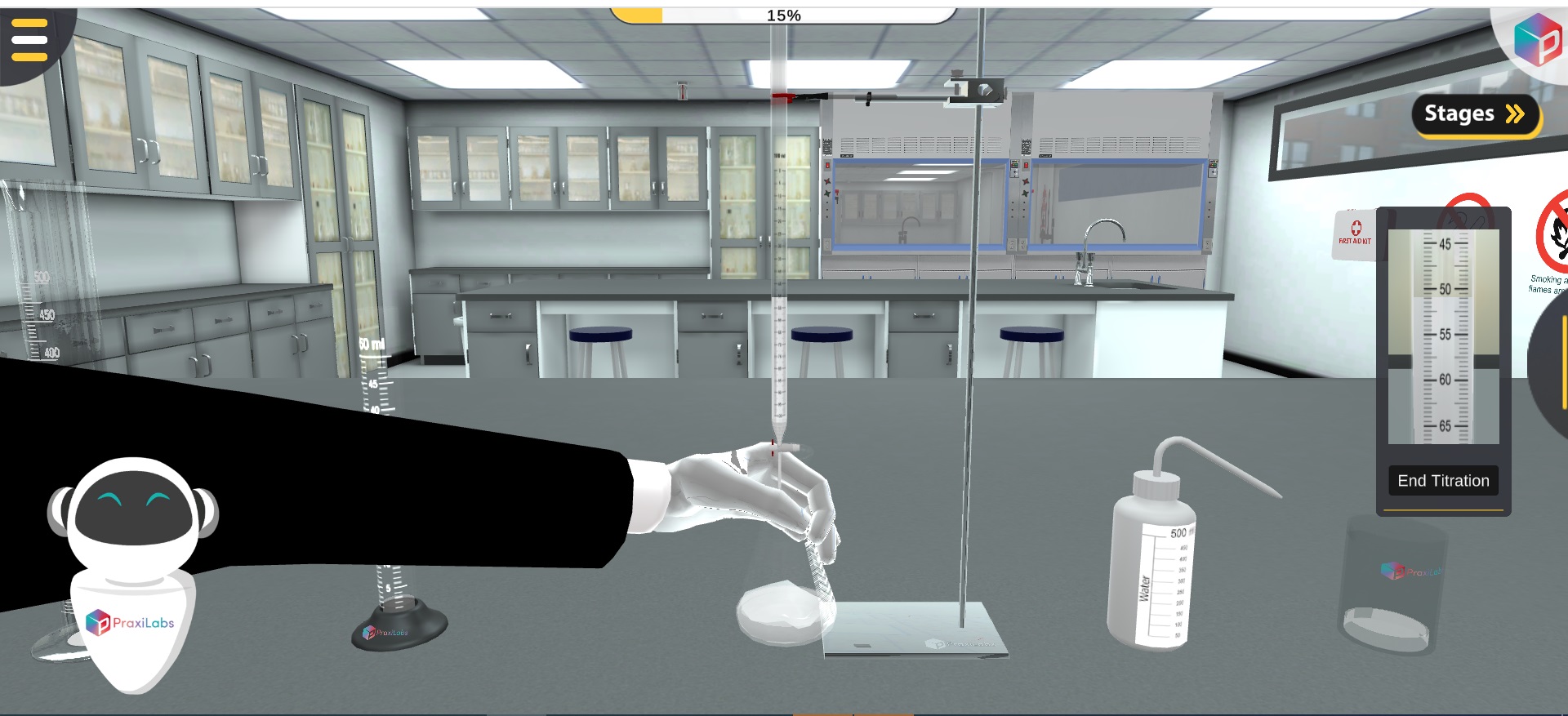
Titration is an analytical technique for quantifying the concentration of a substance (analyte) through a chemical reaction with another substance with a known concentration (titrant) with the aid of indicator colour change.
Simply, the titration is performed by the slow addition of the reactant with the known concentration (titrant) by a burette on the reactant of the unknown concentration (analyte) in an Erlenmeyer flask that contains the indicator.
Titration of a strong acid with a strong base is the simplest type of titrations as both strong acid and strong base (high values of Ka and Kb) completely dissociate in water, resulting in a strong acid-strong base neutralization reaction.
An acid-base titration is utilized to quantify the unknown concentration of an acid or base by neutralizing it with an acid or base of known concentration.
The unknown concentration could be calculated based on the stoichiometry of the reaction.
In the acid base titration virtual lab, the acid-base titration reaction is a neutralization reaction where both materials react to produce salt and water.
For example, hydrochloric acid and sodium hydroxide form sodium chloride and water in HCl NaOH titration:
H + (aq) + Cl - (aq) + Na + (aq) + OH - (aq) →H2O (l) + Na + (aq) + Cl - (aq)
As a result, for a monoprotic acid and base at the endpoint:
Macid *Vacid = Mbase*Vbase
The acid-base titration could be conducted by using a pH indicator or pH meter in the acid base titration virtual lab.
A pH indicator shows the endpoint at which colour change occurs due to pH change.
At this point, the moles of titrant just exceed the moles of the analyte.
A strong acid-strong base titration is performed using phenolphthalein as an indicator in acid base virtual lab setting.
Phenolphthalein is used because it changes colour in a pH range of 8.3 to 10. Phenolphthalein is colourless in acidic pH while appears pink in alkaline pH.
On the other hand, the concentration of unknown acid or base could be also determined by measuring the change in pH of the solution titration to measure the equivalence point.
The equivalence point is the point at which an equivalent number of moles of a base have been added to an acid or vice versa.
Therefore, the equivalence point of the titration is the point at which exactly enough titrant has been added to react with all of the analytes.
At the equivalence point, equal amounts of H+ and OH- ions will combine to form H2O, resulting in a pH of 7.0 (neutral).
The reaction equation is shown below in the net ionic form: H+ (aq) + OH– (aq) → H2O (l)
The stoichiometry of the reaction is one mole of hydrogen ion that reacts with one mole of hydroxide ion to produce one mole of water.
At the end of the experiment, the volume of NaOH at both endpoint (calculated from the pH indicator method using titration simulation) and the equivalence point (calculated from the pH meter method in HCl titration) will be used to calculate the molar concentration of HCl through titration virtual lab techniques and acid base titration simulation platforms.
The process of determining the exact concentration (molarity) of a solution is called standardization.
Afterwards, the concentration of the unknown HCl solution could be determined.