




2.5M+
Active Users Worldwide
80%
Improved Learning Retention
60%
Reduction in Laboratory Costs
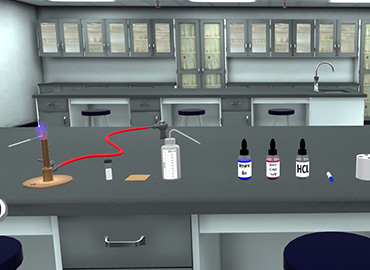
⦁ Place a slide with a heat fixed smear on a staining tray. ⦁ Gently flood the smear with concentrated carbol fuchsin. ⦁ Heat the stain until vapour just begins to rise. N.B. Do not overheat ⦁ Heating the stain: Great care must be taken when heating the carbol fuchsin. Only a small flame should be applied under the slides using an ignited swab previously dampened with a few drops of acid alcohol or 70% ethanol or methanol. ⦁ Do not use a large ethanol-soaked swab because this is a fire risk. ⦁ Allow the heated stain to remain on the slide for 5 minutes. ⦁ Tilt the slide slightly and gently rinse with tap water (or distilled water using a wash bottle if the tap water is not clean) N.B. While washing the slide after staining, do not let the water stream fall directly on the smear. This may disrupt the smear. Let the stream of water flow slowly along the surface, such that only the stain is flooded, and the smear is intact. ⦁ The smear will appear as a red circle on the slide. ⦁ Decolorize using 20% H2SO4 (or 3% HCL). Add the acid and leave it for 1-2 minutes. ⦁ Repeat this step until the smear appears pink in color. Caution: Acid is flammable; therefore use it with care well away from an open flame. ⦁ Immediately rinse with water. ⦁ Flood the smear with methylene blue dye and leave it for 1-2 minutes. ⦁ Tilt the slide slightly and gently rinse with tap water or distilled water using a wash bottle. ⦁ The smear will appear as a blue circle on the slide. ⦁ Blot dry the slide with bibulous paper. ⦁ View the smear using a light-microscope under oil-immersion lens (100x).
To become proficient at performing the Ziehl Neelsen stain consistently and accurately following the ZN staining procedure.




