Last Updated on May 15, 2025 by Muhamed Elmesery
Table of Contents
What is Recrystallization?
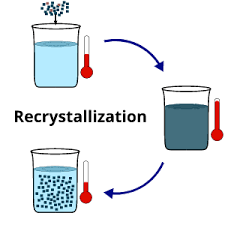
The recrystallization definition is a process used for purifying compounds that contain impurities and these compounds are:
- Dissolved at solvents.
- Solids at room temperature.
- Obtained from chemical reactions or natural sources.
On the other hand, the impurities include mixtures of soluble, insoluble, and colored substances. Each type of these impurities needs to be removed by a separate procedure in the recrystallization process to obtain a pure compound.
As we know what is recrystallization , we will also know in this article the purpose of the recrystallization experiment, types of crystallization, working principle, examples and steps of crystallization, what are the properties of the solvent used in recrystallization, important applications and more.
The Purpose of Recrystallization
The purpose of recrystallization is to separate or remove the impurities in a solid compound that are dissolved in a solvent to obtain a pure compound. And this technique depends on the fact “Solid materials tend to be more soluble in hot liquids than in cold liquids.”
The Recrystallization Principle
The Recrystallization principle depends on solubility: compounds with impurities (called solutes) tend to be more soluble in hot liquids (solvents) than they are in cold solvents.
In the case of a hot saturated solution, if it is allowed to cool, the solute (compound) is no longer soluble in the solvent and the formation of pure compound crystal starts. The impurities are dissolved and excluded from the crystals to form pure solid crystals that can be separated by the process of filtration.
The process of recrystallization can start as a result of:
- Drop in temperature.
- Seeding which means adding a pure crystal.
- Scratching the wall of a glass container may lead to a minute crystal appearance that is called “nucleation”.
The difference between crystals may depend on the environmental conditions, experience, cooling rate, and the solvent nature.
In PraxiLabs virtual chemistry labs, you can instantly track performance and guide science lab simulation practice
Pick The Best Virtual Plan from Praxilabs
Recrystallization Temperature
It is the temperature when the material begins to change its mechanical properties because of the new grains created at the grain boundaries of the already existing grains.
In general, the recrystallization temperature is typically one-third to one-half the melting point, and raises the atomic mobility so the recrystallization begins.
The Recrystallization temperature is about 0.3-0.4 times the pure metals melting point and 0.5 times the alloys melting point.
Recrystallization Steps
Note that in this example, we assume that the present impurities are the soluble ones only.
The 5 main steps of recrystallization procedure are:
1- suitable solvent Detection
Before carrying out the actual steps of the recrystallization process, you must determine the suitable solvent for the impure compound (solute) by doing the solubility tests.
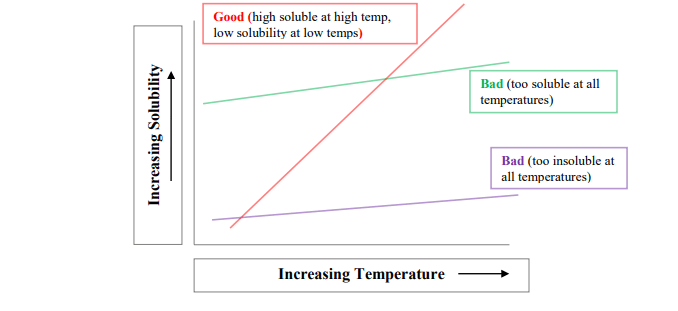
Any compound usually exhibits one behavior of the general three:-
- Compound with high solubility in both cold and hot solvents.
- Compound with low solubility in both cold and hot solvents.
- Compound with low solubility in a cold solvent and high solubility in a hot solvent.
Solvents that show the first two behaviors are not suitable for the recrystallization process. The suitable and optimal solvent that can be used as a recrystallization solvent is the one that exhibits the third behavior, that is, low soluble (or insoluble) at decreased temperatures, high soluble in high temperatures, and sparingly soluble at room temperature.
The following diagram shows the relation between solubility and temperature
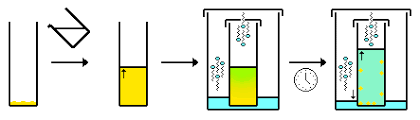
2- Dissolving the Solute in the Suitable Solvent
The next step after determining the suitable solvent is adding the solvent (that is near or at boiling point) to a beaker containing the compound with the impurities. Then, heat the beaker and keep adding the solvent until the solute is dissolved completely.
3- Cooling the Solution
In this step, we will allow the solution to cool slowly, first in the open air, and then allow it to continue cooling in an ice bath. The slow cooling here is important to obtain purer crystals that will precipitate at the bottom of the beaker (The slower the cooling rate, the larger the formed crystals).
Note: The process of “seeding” means the addition of the compound’s pure crystals that forms a surface for the solute for the recrystallization process.
4- Obtaining the Crystals by Filtration
The important and desirable part of the mixture is the pure crystals that form at the bottom of the solution, and so the crystals must be collected and isolated from the solvent by filtration.
“Vacuum filtration” is the common technique used to isolate the remaining crystals in the beaker. By using an aspirator, the suction is created and the process of filtration is done. By using a Buchner funnel, anything remaining in the beaker is poured.
“Gravity filtration” can be used if there are no crystals seen in the beaker. Gravity filtration depends on adding activated carbon to the solution, then letting the new mixture (activated carbon and the solution) boil and by a funnel system, the new mixture is transferred to a new beaker that contains the boiling solvent. The excess carbon is removed by the filter paper in the funnel. After the slow cooling, large crystals should be formed and present at the bottom of the beaker.
5- Allowing the Resulting Crystals to Dry
After rinsing the crystals with a small amount of ice-cold solvent, we will let the crystals dry by-
1- Leaving them in the aspirator.
2- Transferring them to a glass dish to wait a while longer.
Note: We can test the crystal’s purity by conducting a “melting point determination” test.
Types of Impurities
There are three different types of impurities:
- Insoluble Impurities.
- Colored Impurities.
- Soluble Impurities.
Now, we will clarify how to remove each type of the impurities:
Insoluble Impurities: This type can be removed easily by dissolving the compound in a hot solvent, filtering the solution to remove the insoluble impurities (as they are left behind in the filter paper), then evaporating the solvent to obtain the solid compound.
Colored impurities: This type can be removed by the same steps in the case of insoluble impurities, but with an additional step.
The additional step is adding activated charcoal after the compound is dissolved in the solvent. The role of activated charcoal here is to absorb the colored impurities, then it is left behind in the filter paper.
Soluble Impurities: This type can’t be removed by filtration, because its solubility is similar to the solubility of the desired compound.
The soluble impurities can be removed by the following steps:
- Conducting the solubility tests to determine the suitable solvent (low solubility in cold solvent and high solubility in hot solvent).
- Dissolving the desired compound with the soluble impurities in a minimum of near or at boiling solvent.
- Cooling the new solution slowly and without interruption. As a result of cooling the solution, the solubility of (the compound + the soluble impurities) decreases, so the solution becomes saturated with the desired compound, and the process of crystallization begins.
- Only the desired compound crystals form as crystal formation is a highly selective process. On the other hand, the soluble impurities will remain in the solution even after the solution has cooled because they are found in smaller amounts and so the solution is never saturated with the impurities.
- After removing the solvent and the soluble impurities, a final rinse with an ice-cold solvent is done to clean off any residual soluble impurities.
- Finally, after evaporating the solvent, the pure crystals of the desired compound are present.
How Does Recrystallization Work? (Acetanilide Recrystallization)
For more understanding, we will take acetanilide recrystallization and recrystallization of acetophenetidin as examples of how recrystallization work.
The following example depends on the fact that “Solid materials tend to be more soluble in hot liquids than in cold liquids.”
Solubility of Acetanilide in Ethanol
At 0 temperature, acetanilide in ethanol solubility is 18g/100mL and this means at 0 ºC, if we put 50 grams of acetanilide in 100 mL of ethanol, about 18 grams will dissolve in the ethanol and about 32g (the rest) will remain suspended in the solution.
Notice that if we increase the temperature, the solubility also increases.
At 50 ºC, acetanilide in ethanol solubility is 80g/100mL and this means at 50 ºC (we increase the temperature of the same during the acetanilide-ethanol suspension) all of the acetanilide (80 grams) will dissolve in the ethanol. And we can add more acetanilide (more than 80 g) but in this case, we will have a saturated solution as the additional solid of acetanilide would no longer dissolve.
The supersaturated solution is formed when the solution is allowed to cool again to 0 temperature, as more acetanilide is dissolved in ethanol.
Solubility of Acetanilide in Water
At 99 °C, if we dissolve 5 grams of acetanilide in 100 ml of water, and then let the solution cool to 10 °C, we will notice that:
- 4.5 g will form crystals (The recovery would be 90%).
- 0.5 g will remain in the solution (The 10% remaining in the solution would be lost).
In the case of recrystallization of acetophenetidin, the best solvent for acetophenetidin is water because, at boiling point, the solute is completely soluble, but when the solution is placed in an ice bath, the crystals reappear.
PraxiLabs provides virtual labs simulations in Chemistry, Physics and Biology that you can access anytime and anywhere
Start The Virtual Chemistry Labs Experience Now!
Types of Recrystallization
Depending on the condition for the solution and the required solid for recrystallization, there are many different types of recrystallization. Here are the 6 most common types:-
1- Single Solvent Recrystallization
The first type we will talk about is single solvent recrystallization which is also known as ” Normal recrystallization”.
The steps of recrystallization in the single solvent method are:
First, the compound (with the impurity) is solvated into a suitable solvent that should have the ability to dissolve both solute and impurity.
Then, increase the temperature until the saturation point is reached and let the solution cool down.
At a certain temperature, the solute is no longer invisible and its solubility drops down. Finally, by cooling, the crystals start to form and appear clearly.
In the case of ideal conditions, don’t worry about the impurities as their solubility never decreases due to the drop in temperature as the impurities are soluble in the suitable solvent. In reality, this process is repeated several times to achieve good purity.
-
Multi-Solvent Recrystallization
Multi-solvent recrystallization refers to the process of recrystallization that involves more than one.
The steps of recrystallization in the multi-solvent method are:
At a certain temperature, the solution (solute and impurity) is prepared in the first solvent, assuming that each of the impurity and the solute are soluble in that solvent.
Then, the second solvent is added to the solution which makes the solvent able to either dissolve the solute or impurity. This separates impurities from desired solutes one way or the other.
The principle and procedure of both the single-step recrystallization and the multi-step recrystallization processes are the same but the difference is adding a second solvent to the solution (that contains the first solvent, the impurities, and the solute).
-
Hot Filtration Recrystallization
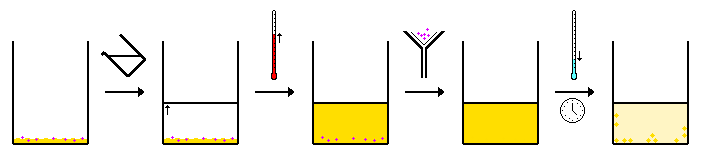
Hot filtration recrystallization is the type that is suitable in the case that insoluble impurities and other materials are present along with the desired solute. But here the simple recrystallization procedure is not enough to achieve the required purity.
The insoluble impurities must be filtered out when the mixture is in the form of a solution and the only thing filterable is the insoluble material. This process depends on using filter paper that serves the purpose but the filtration assembly should be kept warm to make sure that no crystallization of the desired solute happens.
Notice that if the solute were crystallized before the step of passing through the filter paper, it would be lost and the main purpose of the recrystallization procedure would fail.
-
Seeding
Seeding is considered an additional step to the already suitable recrystallization process. In this case, the saturated solution needs an induction to begin the process of crystallization which could be done by adding a seed.
As we mentioned before, seeding means adding a pure crystal of the compound which forms a surface for the solute to crystallize. Only a single crystal of the desired solute or even a dust particle can be used for this purpose.
-
Scratching Glass Container
Scratching glass container is the same as seeding. Scratching glass containers creates vibrations that may lead to the required arrangement of solute molecules together, which leads to stimulate crystallization.
It is suggested that this process is a part of the seeding process because when scratching glass, the small particles of glass that wither away from the glass surface can serve as the sites of seeding and so it stimulates the nucleation to begin.
-
Single perfect crystals
Making perfect crystals has always been a difficult process. The small amounts of solute and the slow crystal development can help to grow and form perfect crystals. For this goal, some techniques are used like:
- Solvent slow evaporation.
- Slow solvent diffusion by applying the mechanism of evaporation condensation to the second solvent (in case of multi-solvent recrystallization).
- Volatile solvent slow evaporation.
- In multistep recrystallization, applying interface delay for the 2 solvents to intermix.
- By solvent delivery or intermixing equipment that is specially designed.
Applications of Recrystallization
There are many useful applications of the recrystallization technique like:
- Purification for crystalline materials.
- Pharmaceutical product development such as paracetamol is purified by the recrystallization process and also by studying the impact of crystal habit on these products.
- Studying compounds’ flowability and compaction in different temperatures and environments.
- Studying the drug mechanism and regeneration.
- Texture controlling for certain crystals.
- Studying certain chemical compounds’ wettability and bioavailability.
- Isomers separation depending on the difference of solubility as a chemical property
- Synthesis of nanoparticles involved in drug delivery systems.
- Comparative study for the solubility of 2 or more solute components for chiral recognition and others.
- Studying nucleation and compaction.
- Polymorphism study, which means studying the ability of a crystal structure to exist in more than one crystal.
Try Praxilabs 3D science Experiments Lab for free now with over 45 different and unique experiments available.
Recrystallization Revealed: Your Top FAQs Answered!
What is recrystallization and its application?
Recrystallization is a process used for purifying compounds that contain impurities which are obtained from chemical reactions or natural sources. The main purpose of recrystallization is to separate or remove the impurities in a solid compound that are dissolved in a solvent to obtain a pure compound.
One application is in pharmaceutical product development; such as paracetamol which is purified by the recrystallization process and also by studying the impact of crystal structure on these products.
What are the 7 steps of recrystallization?
The 7 steps of recrystallization can be summarized as the following:
1. Pick the solvent
The criteria used to choose an appropriate recrystallization solvent includes
- Finding a solvent with a high temperature coefficient.
- Using a solvent that dissolves impurities readily or does not dissolve them at all..
- Ensuring the solvent does not react with the solute.
- Using a solvent that is nonflammable, inexpensive and volatile. Solvents with low boiling points (i.e., volatile) can be easily removed from the resultant crystals by simply allowing the solvent to evaporate.
2. Dissolve the solute
The solute should only dissolve when the solvent is heated. Therefore, the solvent is heated to its boiling point and then slowly added to completely dissolve the solute.
Dissolving the solute generally involves adding a small volume of hot solvent, swirling the flask (or stirring the solution), and watching to see if the solute dissolves.
3. Decolorize the solution
If the solute is supposed to be white in its pure solid state (most organic solids are) and the solution is colored after dissolving all the solute, decolorizing carbon should be added. This step will help cause the colored molecules to adsorb onto the surface of the decolorizing carbon, thereby ridding the solution of these impurities. Should these impurities remain in solution, they may become trapped in the developing crystal during cooling. Review the material about decolorizing carbon.
4. Filter any solids from the hot solution
If decolorizing carbon was used or undissolved impurities remain in the hot solution, it is necessary to use gravity filtration while the solution is still hot. Do not use vacuum filtration with a Buchner funnel under any circumstances,as this can cause premature crystal formation while the solution passes through the vacuum filter.
This leads to premature crystal development as the solution passes through the vacuum filter. (The vacuum reduces the pressure, but also the temperature.) If Impurities become trapped in the crystal lattice and steps 1 through 3 will need repeated!
5. Crystallize the solute
This involves allowing the hot solution with the solute dissolved to return to room temperature slowly. The slower the cooling process, the lower the chance of trapping impurities in the developing crystal lattice. Allow the solution to reach room temperature.
6. Collect and wash the crystals
The resultant crystals formed via this process can be collected by vacuum filtration, provided the solution is at room temperature and no further crystal growth is evident. To ensure all crystals transfer to the Buchner funnel., rinse with a small amount of the cold recrystallization solvent.
7. Dry the crystals
After purification, it’s usually necessary to quickly dry the crystals, especially if a melting point is to be measured immediately This can be done by leaving the crystals in the Buchner funnel and keeping the vacuum on for a few minutes. Alternatively, if more time is allotted, the crystals can be stored safely and allowed to sit for a few days, allowing the solvent to evaporate over time.
What is the principle behind recrystallization?
The main principle for recrystallization is based on dissolving the impure solid compound in hot solvent to form a saturated solution. As this solution cools, the solubility of the compound decreases, and pure crystals from the solution (the solubility of most solids increases with increased temperature).
What is a real life application of recrystallization?
Purification of seawater is a real life application of recrystallization.
Recrystallization is applied in the salt purification process to remove unwanted impurities, aiming to obtain ahight concentration of sodium chloride (NaCl) while minimizing impurities as far as possible.
What are the types of recrystallization?
Here are the most common types of recrystallization:-
- Single Solvent Recrystallization.
- Multi-Solvent Recrystallization.
- Hot Filtration Recrystallization.
- Seeding.
- Scratching Glass Container.
- Single perfect crystals.
Discover Our New Release Experiments in Chemistry
Organic Chemistry
Grignrad Preparation
By the end of Grignrad preparation, your students will be able to:
- Become proficient at running organic chemical reactions.
- Learn the basics of Grignard preparation.
- Understand the mechanism of Grignard reactions.
- Recognize the function of Grignard reactions.
- Gain hands-on training on how the setup of reaction is used.
Preparation of Ethyl Propionate (Propanoic acid ethyl ester) (Fisher Esterification)
Preparation of Ethyl Propionate (Propanoic acid ethyl ester) (Fisher Esterification) simulation will help your students to:
- Become proficient at running organic chemical reactions.
- Learn the basics of organic synthesis procedures.
- Understand the mechanism of the Fischer Esterification reaction.
- Recognize the purpose of the Fischer Esterification reaction.
- Gain practical training on how reflux and the reaction setup are used.
Nitration of Methylbenzoate
By the end of Nitration of Methylbenzoate simulation, your students will be able to:
- Become proficient at running organic chemical reactions
- Learn the basics of organic synthesis procedures.
- Understand the mechanism of electrophilic nitration reactions for aromatic rings.
- Identify the role of Electrophilic substitution reactions.
- Develop skills in synthetic organic chemistry techniques.
Basic Radical Tests
- Test for Unknown Basic Radical 13
- Test for Unknown Basic Radical 14
In our basic radical tests, students will understand various tests to identify the cation present in a given salt, and the chemical reactions that take place during each test. They will also acquire the skills to perform the experiment in a real lab once they understand the different steps in the procedure.
Visit Our Ever-Expanding Catalog of 3D Chemistry Virtual Simulations and Enhance Your Students’ Learning Outcomes!
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science