Last Updated on June 18, 2023 by Sara Assem
Acids and Bases play a vital role in our life and all of the environment around us. When you brush your teeth with toothpaste or wash your hands with soap or take your coffee, you’ve already experienced acids and bases.
In chemistry, substances are classified as acids or bases or neutral, depending on some characteristics like PH and behavior with water. Acids and bases are also sub classified into weak and strong acids and bases, depending on the strength. In this article we will focus on strong acids and bases but first we will clarify some important definitions.
Table of Contents
General Look at Acids and Bases
Before explaining strong acids and bases, we will first clarify what is meant by acids and bases in general and based on:
- Hydrogen ion H+ or hydroxide ion OH- production when dissolved in water.
- Transfer of proton.
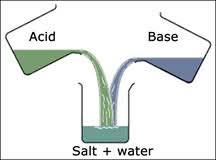
In case of mixing acids and bases, they react together to neutralize the properties and forming a salt and water.
Acids
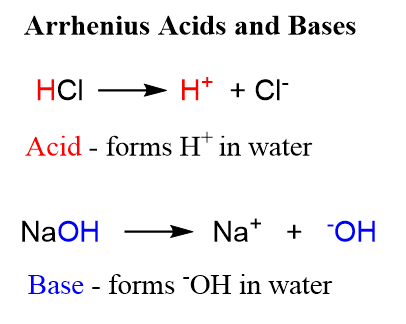
An acid is a matter that produces ions of hydrogen (H+) when dissolved in water. All acids must contain at least one hydrogen ion. Acids are Protons donors.
Ex:- HCl (aq) ———– H+ (aq) + Cl– (aq)
Acids must have acidic protons (H+) to be donated and depending on no of acidic protons, acids can be
– Monoprotic (ex: hydrochloric acid and nitric acid)
– Diprotic (ex: sulfuric acid)
– Triprotic (ex: phosphoric acid)
Water can act as an acid when donates a proton to a base and forms hydroxide ion (OH–).
Note:
Super acid is acid with more acidity than pure sulfuric acid. Some are capable of protonating nearly anything, including hydrocarbons. An example of super acids is fluorosulfonic acid HSO3F.
Bases
A base is a matter that neutralizes acid. Bases split to form ions of hydroxide (OH– ) when dissolved in water ( the base that has been added to water is called an alkaline solution). Bases are protons acceptors and to accept protons, they must have a lone pair.
Ex:- NH3 + H+ —————————– NH4+
Bases can be neutral (ex: ammonia) or negatively charged (ex: hydroxide ion).
Water can act as a base when it accepts a proton from an acid and forms hydronium ion H3O+.
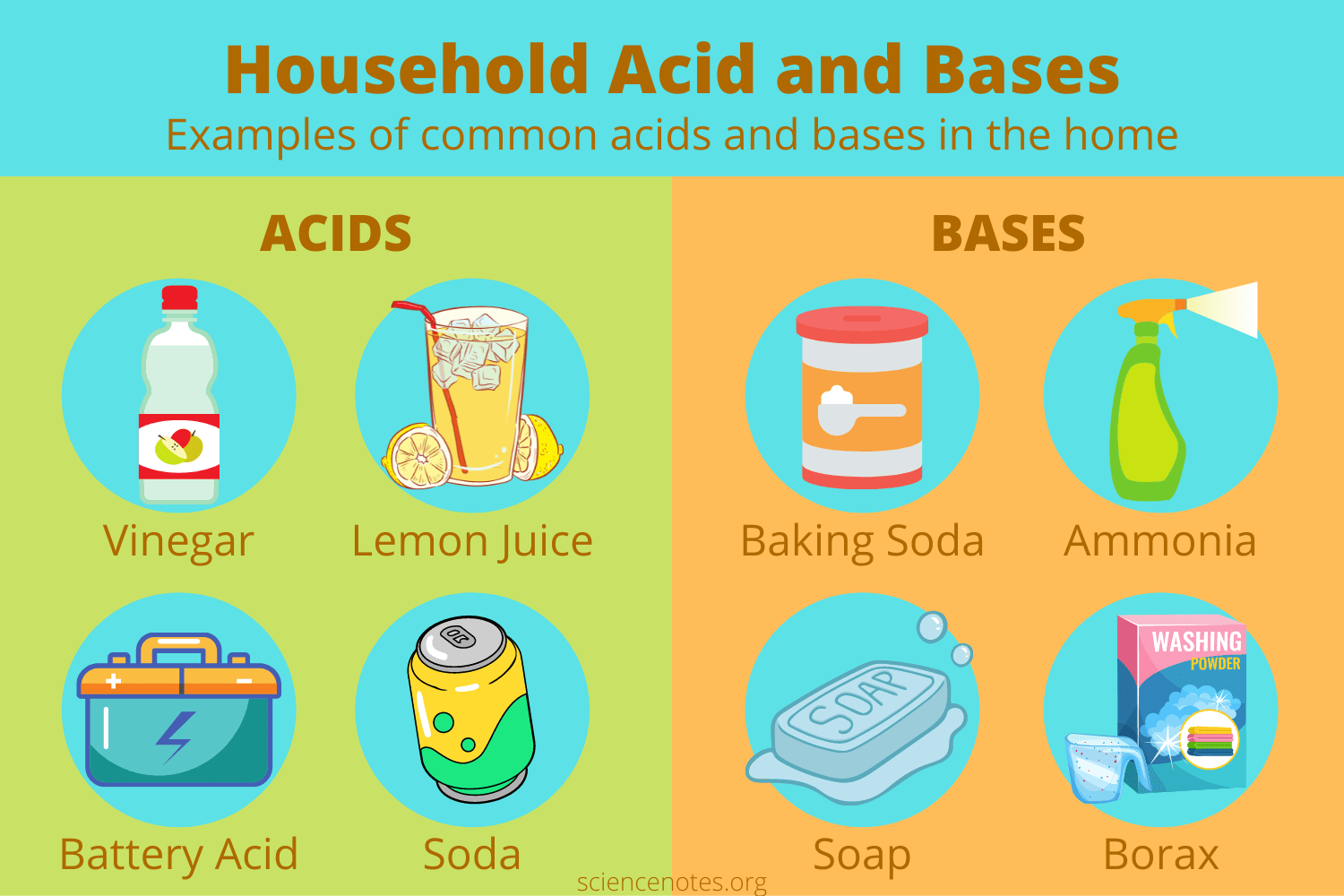
Examples of acids and bases (household):
Properties of Acids and Bases
The following table shows the main properties of acids and bases:
|
Acids |
Bases |
| Acids taste sour. | Bases taste bitter. |
| React with some metals to separate hydrogen. | Bases feel soupy to touch as they can Change the protein structure. |
| React with bases and form salts. | React with acids and form salts. |
| Change the color of litmus paper from blue to red. | Change the color of litmus paper from red to blue. |
| Good electrical current conductor. | Good electrical current conductor. |
| Can destroy cells. | Strong bases can destroy the skin proteins and cause severe burns. |
| Can catalyze chemical reactions. | Corrosive substances that can damage other substances. |
| Soluble in water. | Some bases are soluble in water but others are not. |
| Mineral acids are colorless and in solid case, organic acids are white. | Most of bases are colorless. |
| Commonly used in car batteries and fertilizers. | Commonly used in several cleaning products. |
| Examples: Vinegar (acetic acid) / Hydrochloric acid / Sulfuric acid | Examples: Ammonia / Sodium hydroxide / Potassium hydroxide |
Difference between Acids and Bases
The main difference between acids and bases is the PH value of each of them. The acids pH are below 7 and the bases pH are above 7.
The pH or potential of hydrogen is a measurement for the acidity or alkalinity of a solution on a logarithmic scale on which 7 is neutral, higher values are more alkaline and lower values are more acidic. The pH is equal to −log10 c, where c refers to the hydrogen ion concentration in moles per litre.
Acids and Bases Identification and Measurement
When we test a matter with a pH meter, we get a number range from zero to 14. This is a pH scale, and it can be used to compare substances, also identify and measure acids and bases. note that the pH scale is logarithmic which means that 1 decrease in the pH scale can cause 10 times increase of hydrogen ions concentration.
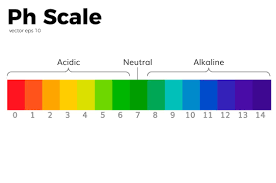
Acids have a pH value below 7. The more H+ ions, the more the acidity of it and the lower the pH value will be. Bases have a pH value above 7. When there is a balance of H+ and OH– ions, it is called neutral and pH value is 7.
For very strong acids, the pH value can be less than zero and for very strong bases, the pH value can be more than 14.
The PH Scale
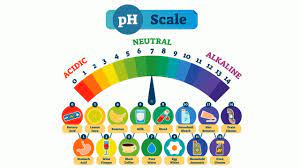
- pH 0 – 2: strong acid
- pH 3 – 6: weak acid
- pH 7: neutral
- pH 8 – 11: weak alkali
- pH 12 – 14: strong alkali
In PraxiLabs virtual labs, you can do any experiment anytime you want easily. Create your free account now and go beyond the learning outcomes you’re looking for!
Acids and Bases Examples
The following table shows list of acids and bases
|
Acids |
Bases |
| H2SO4 – Sulfuric acid | KOH – Potassium hydroxide |
| HCl – Hydrochloric acid | NaOH – Sodium hydroxide |
| CH3COOH – Acetic acid | Li(OH)2 – Lithium hydroxide |
| HNO3 – Nitric acid | Ca(OH)2 – Calcium hydroxide |
| HBr – Hydrobromic acid | Ba(OH)2 – Barium hydroxide |
| H2CO3 – Carbonic acid | C5H5N – Pyridine |
| HI – Hydroiodic acid | HCO3 – Bicarbonate ion |
| H2CO3 – Carbonic acid | NH3 – Ammonia |
Strong Acids and Bases
After understanding the general acids and bases differences, now we will clarify what is meant by strong acids and bases and their general properties.
Strong acids or alkalis are nearly or completely ionised in water. Examples of strong acids (hydrochloric acid and sulphuric acid) / examples of Strong alkalis (sodium hydroxide and potassium).
Strong Acids
Strong acid is characterized by their ability to be completely ionized or dissociated in the solvents. The pKa value of strong acids are less than -2.
Note: when strong acids become more concentrated, they may be unable to fully dissolve. A strong acid is fully dissociated in solutions of 1.0 M or lower concentration.
The general form of the dissociation or ionization reaction of the strong acids is:
HA + S ↔ SH+ + A-
Where:
S is the solvent molecule like water
For example, hydrochloric acid dissociation in water:
HCl(aq) → H+(aq) + Cl–(aq)
Ka is the acid dissociation constant and it measures how completely an acid dissociates in a solution. The large value of Ka indicates that the acid is highly dissociated into its ions and it is a strong acid. Strong acids have a small value of Ka as they completely dissociate in water.
There is a direct relationship between Ka and pKa (the logarithmic acid dissociation constant). Therefore, the stronger the acid the lower the pKa value.
pKa = -log [Ka]
The value of pKa of an acid depends on the solvent. For example, the pKa value of hydrochloric acid is about -5.9 in water and in dimethyl sulfoxide is about -2.0, while the pKa value of hydrobromic acid is about -8.8 in water and in dimethyl sulfoxide is about -6.8.
Strong Bases
Strong base is characterized by their ability to be completely ionized or dissociated into their respective ions.
Most common strong bases are metal hydroxides as they are composed of a metal ionically bonding with a hydroxyl ion OH−, like potassium hydroxide KOH.
Strong bases are considered as good acceptors of protons that cannot remain in aqueous solution. For instance, all oxygen ions are converted to hydroxide ions by accepting protons from water H2O molecules. As a result, water molecules are converted to hydroxide OH–.note that the strong bases cations are soluble in water (if a cation is soluble in water, it can form a strong base).
Ex: BOH + H2O → B+(aq) + OH–(aq)
The potential of hydroxide ions or pOH is a measurement of the concentration of hydroxide ion (OH-). The higher the hydroxide ions concentration in the solution, the lower the value of pOH therefore, a stronger base.
pOH = -log [OH–]
Strong bases commonly have a pH value between 13 \ 14.
Kb or the base dissociation constant measures how completely a base ionizes or dissociates in a solution. The large value of Kb indicates a strong base and it also means the base is highly dissociated into its ions.
There is a direct relationship between Kb and pKb (the logarithmic base dissociation constant). Therefore, the stronger the acid the lower the pKb value.
pKb = -log [Kb]
The following equation shows the relation between pH and pOH of an aqueous solution:
pH + pOH = 14
If either the pOH or the pH value of a solution is known, you can calculate the other.
Examples of Acids and Bases
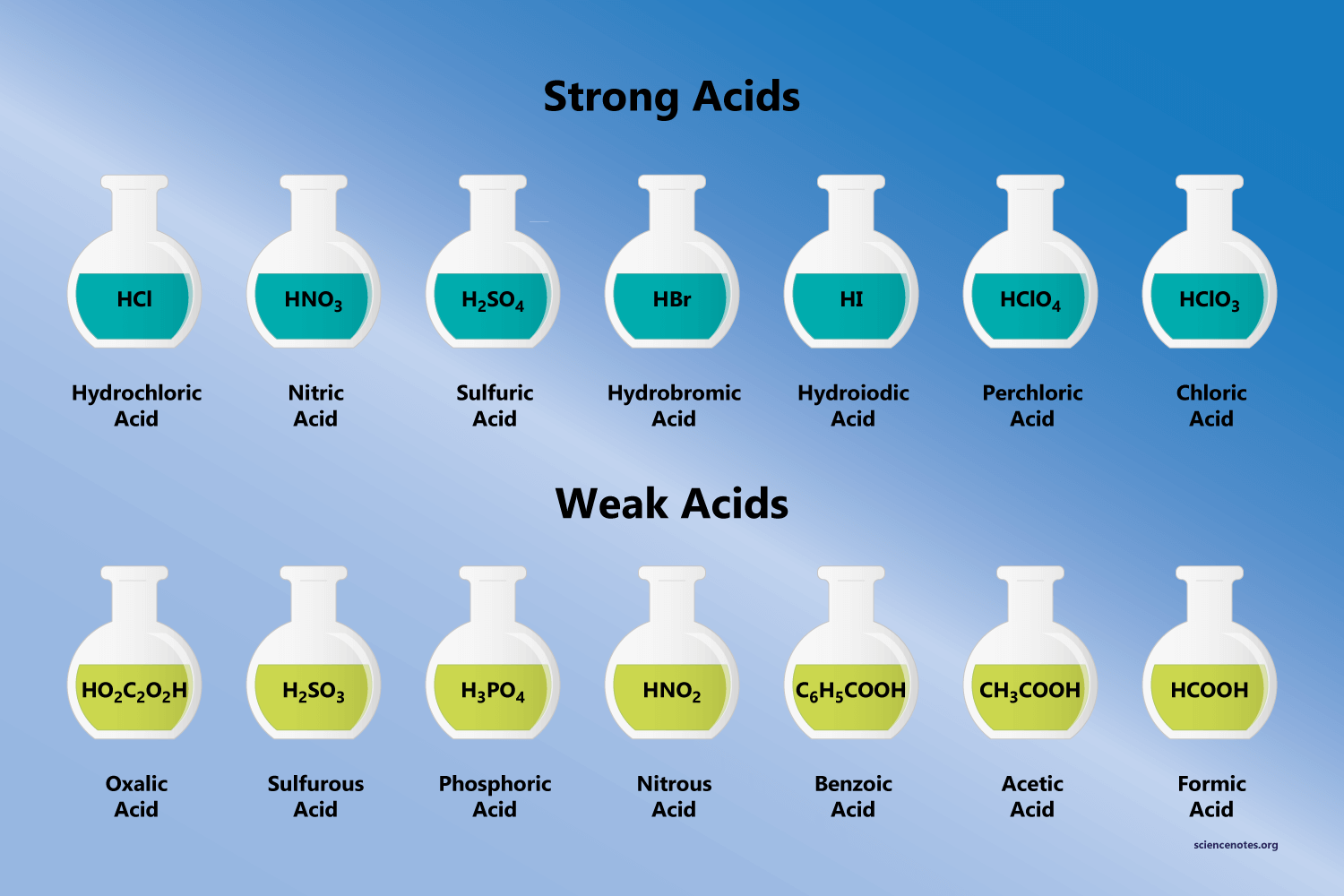
List of Strong Acids and Bases
There are examples of common strong acids and strong bases.
Strong Acids:
- H2SO4 (sulfuric acid)
- HNO3 (nitric acid)
- HCl (hydrochloric acid)
- HI (hydroiodic acid)
- HBr (hydrobromic acid)
- HClO3 (chloric acid)
- HClO4 (perchloric acid)
- Triflic acid (H[CF3SO3])
- Fluoroantimonic acid (H[SbF6]).
Strong Bases:
- KOH (potassium hydroxide)
- LiOH (lithium hydroxide)
- NaOH (sodium hydroxide)
- Ca(OH)2 (calcium hydroxide)
- Ba(OH)2 (barium hydroxide)
- RbOH (rubidium hydroxide)
- Sr(OH)2 (strontium hydroxide)
- CsOH (cesium hydroxide)
PraxiLabs provides virtual lab simulations in Chemistry, Physics and Biology that you can access anytime and anywhere .. Subscribe and get start now!
Strong Acids and Bases Chart
| Acid | Base | |||||
|---|---|---|---|---|---|---|
| Strength of Acid | Ka | Name | Formula | Formula | Name | Strength of Base |
| Strongest | Large | Perchloric acid | HClO4 | ClO4– | Perchlorate ion |
Weakest |
| 3.2 * 109 | Hydroiodic acid | HI | I- | Iodide | ||
| 1.0 * 109 | Hydrobromic acid | HBr | Br- | Bromide | ||
| 1.3 * 106 | Hydrochloric acid | HCl | Cl- | Chloride | ||
| 1.0 * 103 | Sulfuric acid | H2SO4 | HSO4– | Hydrogen sulfate ion |
||
| 2.4 * 101 | Nitric acid | HNO3 | NO3– | Nitrate ion | ||
| ——– | Hydronium ion | H3O+ | H2O | Water | ||
| 5.4 * 10-2 | Oxalic acid | HO2C2O2H | HO2C2O2– | Hydrogen oxalate ion |
||
| 1.3 * 10-2 | Sulfurous acid | H2SO3 | HSO3– | Hydrogen sulfite ion |
||
| 1.0 * 10-2 | Hydrogen sulfate ion | HSO4 – | SO4 2- | Sulfate ion | ||
| 7.1 * 10-3 | Phosphoric acid | H3PO4 | H2PO4– | Dihydrogen phosphate ion |
||
| 7.2 * 10-4 | Nitrous acid | HNO2 | NO2– | Nitrite ion | ||
| 6.6 * 10-4 | Hydrofluoric acid | HF | F- | Fluoride ion | ||
| 1.8 * 10-4 | Methanoic acid | HCO2H | HCO2– | Methanoate ion |
||
| 6.3 * 10-5 | Benzoic acid | C6H5COOH | C6H5COO- | Benzoate ion | ||
| Medium | 5.4 * 10-5 | Hydrogen oxalate ion | HO2C2O2– | O2C2O2 2- | Oxalate ion | Medium |
| 1.8 * 10-5 | Ethanoic acid | CH3COOH | CH3COO | Ethanoate (acetate) ion |
||
| 4.4 * 10-7 | Carbonic acid | H2CO3 | HCO3 – | Hydrogen carbonate ion |
||
| 1.1 * 10-7 | Hydrosulfuric acid | H2S | HSO3– | Hydrogen sulfide ion |
||
| 6.3 * 10-8 | Dihydrogen phosphate ion | H2PO4 – | HPO4 2- | Hydrogen phosphate ion |
||
| 6.2 * 10-8 | Hydrogen sulfite ion | HS- | SO32- | Sulfite ion | ||
| 2.9 * 10-8 | Hypochlorous acid | HClO | ClO– | Hypochlorite ion |
||
| 6.2 * 10-10 | Hydrocyanic acid | HCN | CN– | Cyanide ion | ||
| 5.8 * 10-10 | Ammonium ion | NH4 + | NH3 | Ammonia | ||
| 5.8 * 10-10 | Boric acid | H3BO3 | H2BO3– | Dihydrogenborate | ||
| 4.7 * 10-11 | Hydrogen carbonate ion | HCO3 – | CO3 2- | Carbonate ion |
||
| 4.2 * 10-13 | Hydrogen phosphate ion | HPO4 2- | PO4 3- | Phosphate ion |
||
| 1.8 * 10-13 | Dihydrogen borate ion | H2BO3 – | HBO3 2- | Hydrogen borate ion |
||
| 1.3 * 10-13 | Hydrogen sulfide ion | HS- | S 2- | Sulfide ion | ||
| 1.6 * 10-14 | Hydrogen borate ion | HBO3 2- | BO3 3- | Borate ion | ||
| Weakest | – | Water | H2O | OH- | Hydroxide | Strongest |
The previous table shows the strong acids and bases strength chart.
Source: Merck
Properties of Strong Acids and Bases
Strong Acids
The most common properties of strong acids are:
- They can release H+ ions in aqueous solutions (Arrhenius acid).
- They donate a proton (H+) to a base (Bronsted-Lowry acid).
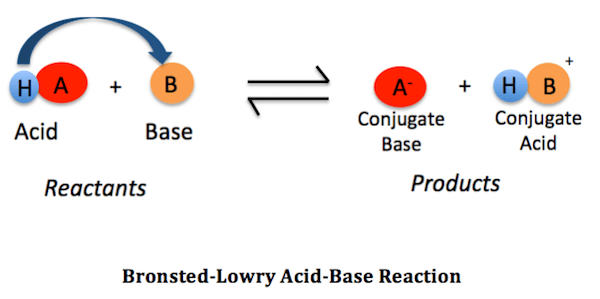
- They are able to receive electrons from a base (Lewis acid).
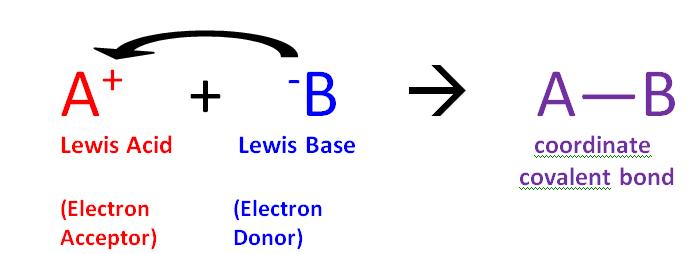
- Can catalyze chemical reaction.
- able to conduct electricity.
- The hydronium ions concentration is equal to the concentration of the acid.
- They are highly reactive and dangerous, so they must be handled carefully.
Strong Bases
The most common properties of strong bases :
- They are good proton (hydrogen ion) acceptors and electron donors.
- They can deprotonate weak acids.
- Their aqueous solutions are slippery and soapy.
- They are less reactive and violent than acids.
- They undergo many chemical reactions, especially with organic compounds (ex: the most common reaction is saponification).
Difference between Strong Acids and Bases
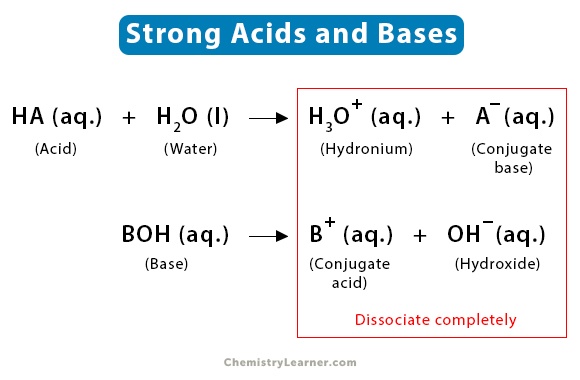
The main difference between strong acids and bases is that after the complete dissolving, strong acids give more hydrogen ions and the strong bases give more hydroxide ions in the solutions.
Weak Acids and Bases
weak acids and bases are partially ionised in water. Examples of weak acids (citric acid and carbonic acid) / examples of weak alkalis (copper hydroxide and ammonia).
when weak acids are dissolved in water, an equilibrium is happened between the weak acid concentrations and its constituent ions, so we can said that weak acids are not completely ionized in solution.
For example, hydrofluoric acid HF is considered as weak acid. When dissolved in water, its ions exist in equilibrium with hydrogen ions, which reacts with water to form hydronium, and flour ions. because the acids don’t completely dissociate into their ionic components, they are called weak acid in this case.
HA + H2O ⇌ H3O+ + A–
The concept is the same in case of weak base; but, in weak base case, we can define a base that cannot completely ionize or accept hydrogen ions which found in the solution.
The base needs to be dissolved in solution to react with the acid and form an acid base pair. note that the base should break into its ions to react in the way it must.
When weak base dissolved in water, the solution so, in this case we said that the weak base does not fully ionize as when it dissociated in water, the result solution contains a small amount of OH ions and a big amount of the nondissociated base.
Ex: the counterpart’s of the base in ammonium hydroxide (NH4OH) are ion of ammonia NH4+ and hydroxide ion OH–; the ion of ammonia NH4+ accepts the acidic hydrogen in solution; but, in case of weak base these ion do not dissolve completely so there is a mixture of the acid base pair and ammonium hydroxide NH4OH.
B + H2O ⇌ BH+ + OH–
Try PraxiLabs Virtual Chemistry experiments lab for free now with over 45 different and unique experiments available.
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





