Last Updated on April 17, 2024 by Zinab Hasssan
Western blot technique is a staple in the toolkit of researchers and scientists, yet it often raises many questions. Whether you’re a student, researcher or a newcomer to the field, understanding the ins and outs of Western blot is crucial for obtaining reliable results.
In this blog, we’ll delve into the most common questions surrounding Western blot test and provide comprehensive answers backed by scientific insights and practical tips. From troubleshooting unexpected results to mastering the intricacies of antibody selection, we’ve got you covered.
Table of Contents
What is Western Blot test?
Western blot or “protein immunoblot” or “western blotting” is an analytical technique used mainly in molecular biology and immunogenetics where antibodies are used to specifically detect their antigen.
It is mainly used to identify certain proteins in a sample. Also, it provides information about protein size and relative abundance in the sample.
The term “blot” or “blotting” refers to the transfer of biological samples from a gel to a microporous membrane and their subsequent detection on the surface of the membrane.
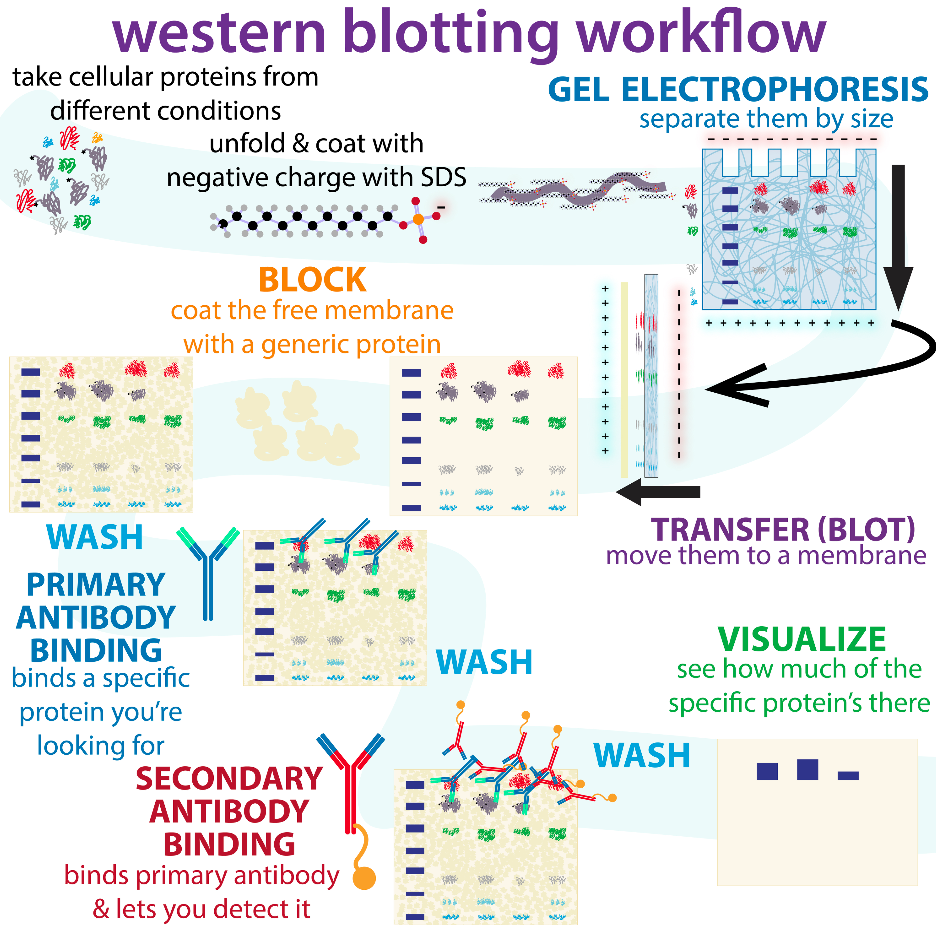
How does Western Blotting work?
In the Western blot technique, a mixture of proteins or nucleic acid is separated depending on specific factors such as molecular weight, thereby categorizing them by type, using gel electrophoresis. These results are then transferred to a microporous membrane producing a band for each protein. The membrane is then incubated with antibodies labeled specifically for the protein of interest.
For more information about Western Blotting steps and processes, check our blog article “The Concept, Processes, and Applications of Western Blotting”
What are the applications of Western Blot?
Western blot method has several applications in scientific and clinical disciplines:
- Identification of protein targets
- Protein interactions
- Epitope mapping
- Protein isoform detection
- Characterization of antibodies
- Subcellular localization
- Post-translational modification
- Autoimmune disorders detection
- Diagnosis for HIV (Human Immunodeficiency Virus) infection, BSE (Bovine Spongiform Encephalopathy, also known as “mad cow disease”), FIV (Feline Immunodeficiency Virus), HBV (Hepatitis B Virus) infection
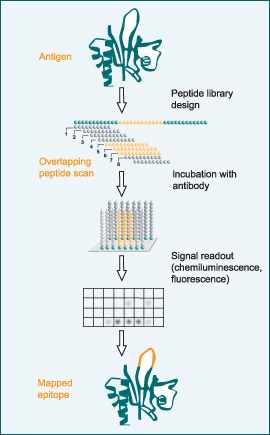
What are primary and secondary antibodies in Western Blot test?
In the Western blot technique, the primary and secondary antibodies play important roles in determining specific proteins from a complex mixture of proteins extracted from cells.
Primary antibodies:
The primary antibody recognizes a specific protein or epitope on a group of proteins, and it is used to check the blocked membrane in Western blot.
When choosing a primary antibody, it is very important to consider the antigen to be detected and the availability of suitable antibodies for that antigen. Not all primary antibodies are suitable for Western blot, so their application should be verified before use.
Secondary antibodies:
The secondary antibody is used in the detection, sorting, or purification of target antigens by binding to the primary antibody, which directly binds to the target antigen.
They are directed against the species of the primary antibody, and they should be raised in a species different from the host species of the primary antibody.
For more information about Western Blot analysis, check our blog article “How to Analyze Western Blot Data”
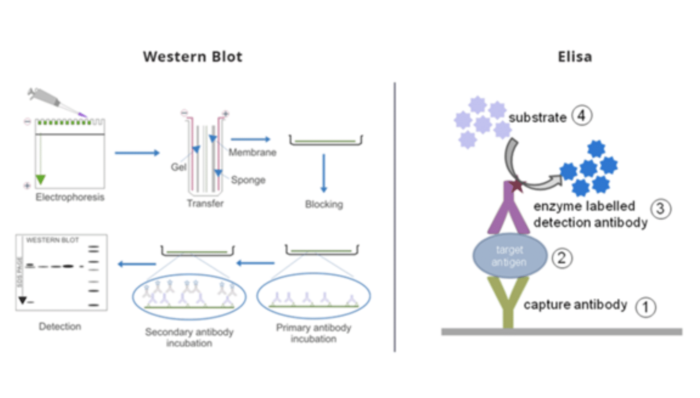
What is the difference between Elisa and western blotting?
Let’s compare Western blot and ELISA tests.
| Elisa | Western Blot |
| The ELISA test uses enzymes or antibodies attached to a solid surface to create the test surface. A sample is then added to the test surface. Antibodies or enzymes reacting or attaching to proteins indicate a positive result. | The Western blot test is performed after the gel-electrophoresis. The separated proteins are transferred (or blotted) onto nitrocellulose or nylon membranes and identified by specific antibodies that are tagged by a secondary protein. |
| It is a related technique, but instead of using antibodies to detect virus antigens, it uses virus antigens to detect antibodies. A positive ELISA indicates the presence of an antibody to a virus in our patient.
That patient may have had a viral infection to which their immune system has responded. Often, this will mean they have no live virus and will have recovered. Since antibodies can persist for a while after a virus infection has occurred, ELISA can detect infections that have occurred a while ago.
|
It detects viral antigens (proteins usually on the surface of viruses) using antibodies against those proteins. A positive Western blot test indicates the presence of viral antigen – which often means live virus in our patient. That patient may have an ongoing viral infection.
|
How do we troubleshoot Western Blot test problems?
Although the Western blot test is simple, it can be prone to various issues that may result in false results. These problems can typically be categorized into five main areas:
- Unusual or unexpected bands
- No bands
- Faint bands or weak signal
- High background on the blot
- Patchy or uneven spots on the blot.
Unusual or unexpected bands may result from protease degradation, leading to bands appearing at unexpected positions. In such cases, it is advisable to use a fresh sample that has been stored on ice or to consider modifying the antibody used. If the protein appears to migrate to a higher position than anticipated, reheating the sample can assist in disrupting the quaternary protein structure.
Similarly, blurry bands are often the result of high voltage or the presence of air bubbles during the transfer process. To address this issue, it is essential to ensure that the gel is run at a lower voltage and that the transfer sandwich is properly prepared. In addition, changing the running buffer may also alleviate the problem. Nonflat bands can occur when the sample travels too quickly through the gel due to low resistance. To fix this, the gel should be optimized to fit the specific sample being analyzed. Finally, white (negative) bands observed on the film typically indicate an excess of protein or antibody.
The absence of bands can also stem from various factors related to the antibodies, antigens, or buffers used. If an improper antibody, either primary or secondary, is used, the expected bands may not appear. In addition, the concentration of the antibody must be appropriately adjusted; if it is too low, the signal may not be visible. It’s important to remember that some antibodies are not suitable for Western blot applications and should not be used.
Similarly, weak signals can be caused by low concentrations of antibody or antigens. Increasing exposure time can also help make the band clearer. Another reason could be nonfat dry milk masking the antigen. In this case, use BSA or reduce the amount of milk used.
A high background is often caused by too high a concentration of the antibody, which can bind to PVDF membranes. Another problem could be the buffers, which may be too old. Increasing the washing time can also help decrease the background. Additionally, too high of exposure can also lead to this problem. Therefore, it is advisable to check different exposure times to achieve an optimal duration.
Patchy and uneven spots on the blot are usually caused by improper transfer. If there are air bubbles trapped between the gel and the membrane, it will appear darker on the film. It is also important to use a shaker for all incubations, so that there is no uneven agitation during the incubation. Once again, thorough washing is crucial to remove background noise effectively.
This problem can also be caused by antibodies binding to the blocking agents; in this case, another blocking agent should be tried. Filtering the blocking agent can also help remove some contaminants. Finally, this problem can also be caused by aggregation of the secondary antibody; in this case, the secondary antibody should be centrifuged and filtered to remove the aggregated.
Try Western blot Simulation from PraxiLabs
Western blot test in virtual labs from PraxiLabs aims to teach students how to detect a specific protein in a sample.
By the end of the Western blot virtual experiment, students will be able to:
- Detect a specific protein in a sample using western blot technique.
- Identify the theory behind western blot technique.
- Fully comprehend the steps of Western blot test.
- Design a complete western blot experiment.
- Analyze the visualized protein and understand Western blotting application.
Create your free account and try our simulations now!
 PraxiLabs A virtual world of science
PraxiLabs A virtual world of science





